NDC Code(s) : 43598-224-14, 43598-224-01, 43598-224-05, 43598-219-14, 43598-219-01, 43598-223-50, 43598-223-51, 43598-223-52, 43598-207-50, 43598-207-51, 43598-207-52
Packager : Dr Reddys Laboratories Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| AMOXICILLINamoxicillin TABLET, FILM COATED | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| AMOXICILLINamoxicillin TABLET, FILM COATED | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| AMOXICILLINamoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| AMOXICILLINamoxicillin POWDER, FOR SUSPENSION | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
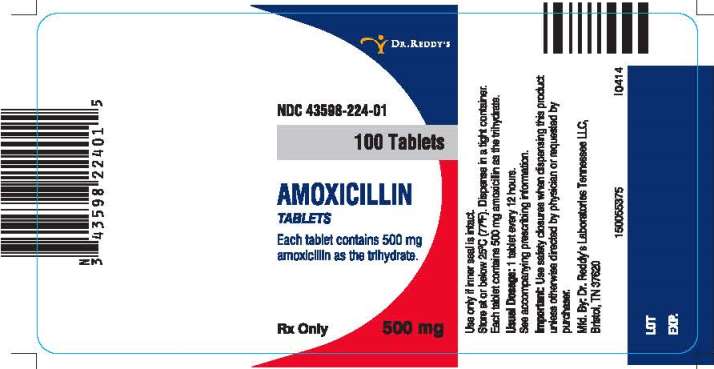
PRINCIPAL DISPLAY PANEL
NDC 43598-224-01
100 Tablets
AMOXICILLIN
TABLETS
Each Tablet contains 500 mg amoxicillin as the trihydrate.
500 mg
Rx Only
Use only if inner seal is intact.
Store at or below 25°C (77°F).
Dispense in a tight container.
Each tablet contains 500 mg amoxicillin as the trihydrate.
Usual Dosage: 1 tablet every 12 hours.
See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physician or requested by purchaser.
Manufactured. By: Dr. Reddy’s Laboratories Tennessee LLC.
Bristol, TN 37620
I0414
150055375
PRINCIPAL DISPLAY PANEL
NDC 43598-219-01
100 Tablets
AMOXICILLIN
TABLETS
Each Tablet contains 875 mg amoxicillin as the trihydrate
875 mg
Rx Only
Use only if inner seal is intact.
Store at or below 25°C (77°F). Dispense in a tight container.
Each tablet contains 875 mg amoxicillin as the trihydrate.
Usual Dosage: 1 tablet every 12 hours.
See accompanying prescribing information.
Important: Use safety closures when dispensing this product unless otherwise directed by physician or requested by purchaser.
Manufactured. By: Dr. Reddy’s Laboratories Tennessee LLC.
Bristol, TN 37620
I0414
150055373

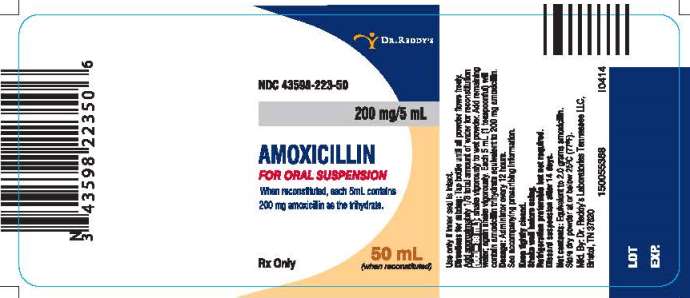
PRINCIPAL DISPLAY PANEL
NDC 43598-223-50
200 mg/5 mL
AMOXICILLIN
FOR ORAL SUSPENSION
When reconstituted, each 5mL contains 200 mg amoxicillin as the trihydrate.
50 mL (when reconstituted)
Rx Only
Directions for mixing:
Tap bottle until all powder flows freely. Add approximately 1/3 total amount of water for reconstitution (total=39 mL); shake vigorously to wet powder. Add remaining water; again shake vigorously. Each 5 mL (1 teaspoonful) will contain amoxicillin trihydrate equivalent to 200 mg amoxicillin.
Dosage: Administer every 12 hours.
See accompanying prescribing information.
Keep tightly closed.
Shake well before using.
Refrigeration preferable but not required.
Discard suspension after 14 days.
Use only if inner seal is intact.
Net contents: Equivalent to 2.0 grams amoxicillin.
Store dry powder at or below 25°C (77°F).
Manufactured. By: Dr. Reddy’s Laboratories Tennessee LLC.
Bristol, TN 37620
I0414
150055388
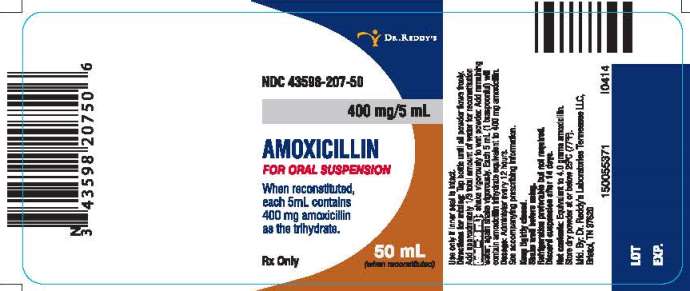
PRINCIPAL DISPLAY PANEL
NDC 43598-207-50
400 mg/5 mL
AMOXICILLIN
FOR ORAL SUSPENSION
When reconstituted, each 5mL contains 400 mg amoxicillin as the trihydrate.
50 mL (when reconstituted)
Rx Only
Directions for mixing:
Tap bottle until all powder flows freely. Add approximately 1/3 total amount of water for reconstitution (total=36 mL); shake vigorously to wet powder. Add remaining water; again shake vigorously. Each 5 mL (1 teaspoonful) will contain amoxicillin trihydrate equivalent to 400 mg amoxicillin.
Dosage: Administer every 12 hours.
See accompanying prescribing information.
Keep tightly closed.
Shake well before using.
Refrigeration preferable but not required.
Discard suspension after 14 days.
Net contents: Equivalent to 4.0 grams amoxicillin.
Store dry powder at or below 25°C (77°F).
Manufactured. By: Dr. Reddy’s Laboratories Tennessee LLC.
Bristol, TN 37620
I0414
150055371