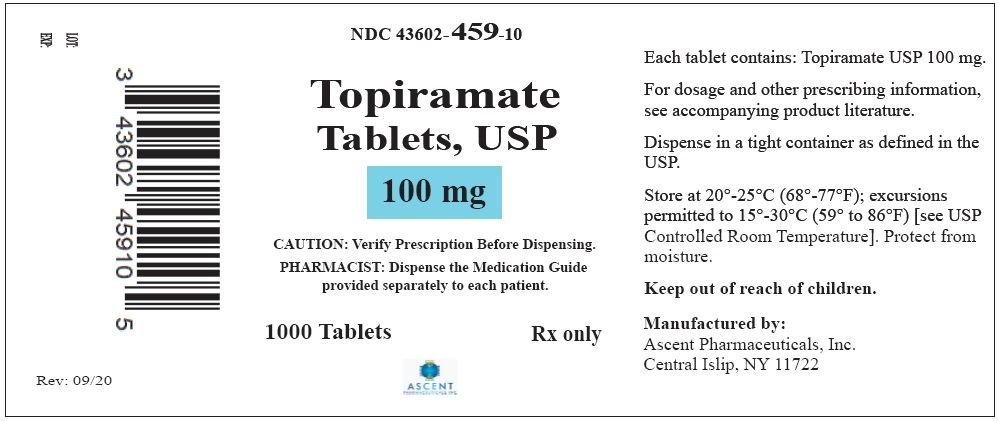
NDC Code(s) : 43602-457-30, 43602-457-10, 43602-458-30, 43602-458-10, 43602-459-30, 43602-459-10, 43602-460-30, 43602-460-10
Packager : Ascent Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TOPIRAMATETopiramate TABLET | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| TOPIRAMATETopiramate TABLET | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| TOPIRAMATETopiramate TABLET | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| TOPIRAMATETopiramate TABLET | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Ascent Pharmaceuticals, Inc.(080938961) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Ascent Pharmaceuticals, Inc. | 080938961 | manufacture(43602-457, 43602-458, 43602-459, 43602-460), analysis(43602-457, 43602-458, 43602-459, 43602-460), pack(43602-457, 43602-458, 43602-459, 43602-460) | |
PRINCIPAL DISPLAY PANEL