NDC Code(s) : 44183-400-22, 44183-180-30, 44183-180-02
Packager : Macoven Pharmaceuticals
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ceftibutenceftibuten dihydrate CAPSULE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| CeftibutenCEFTIBUTEN DIHYDRATE SUSPENSION | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
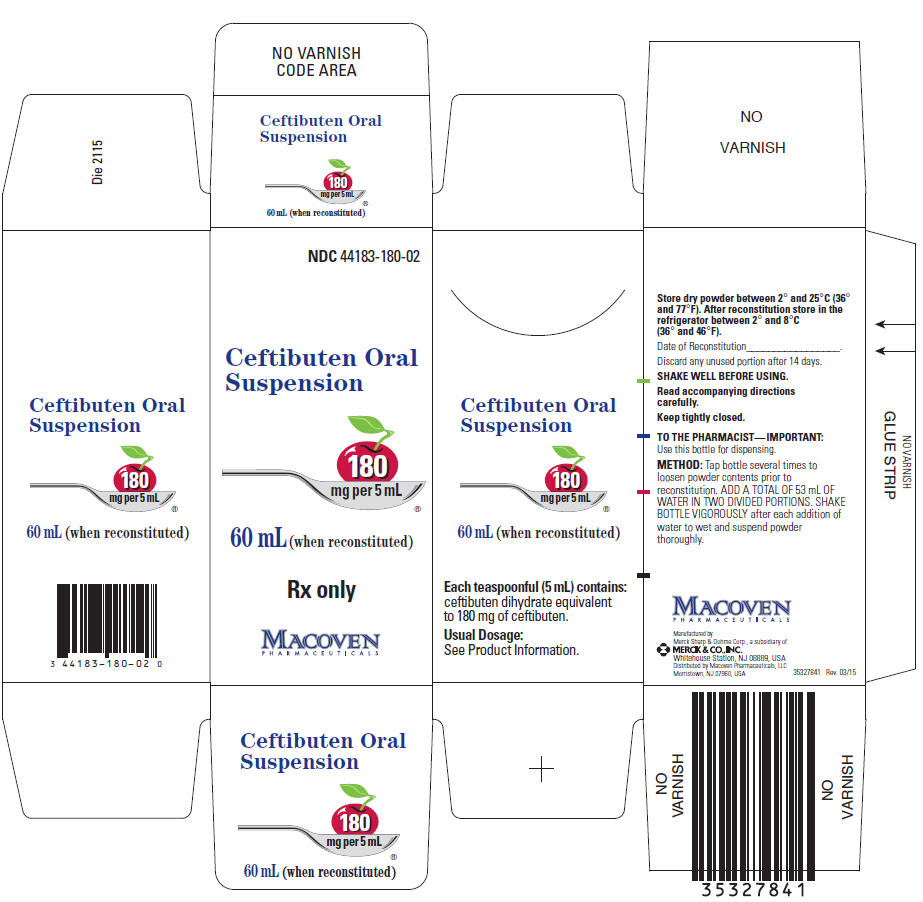
PRINCIPAL DISPLAY PANEL
NDC 44183-400-22
Ceftibuten
Capsules
400 mg
For Oral Administration
Rx only
20 Capsules
MACOVEN
PHARMACEUTICALS

PRINCIPAL DISPLAY PANEL
NDC 44183-180-02
Ceftibuten Oral
Suspension
180
mg per 5 mL
60 mL (when reconstituted)
Rx only
MACOVEN
PHARMACEUTICALS