NDC Code(s) : 45567-0030-1
Packager : Sun Pharmaceutical Industries, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Kit for the Prepartion of Technetium Tc99m Sulfur ColloidTechnetium Tc 99m Sulfur Colloid KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LABELER - Sun Pharmaceutical Industries, Inc.(139261648) |
| REGISTRANT - Sun Pharmaceutical Industries, Inc.(139261648) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sun Pharmaceutical Industries, Inc. | 139261648 | ANALYSIS(45567-0030), LABEL(45567-0030), PACK(45567-0030), MANUFACTURE(45567-0030) | |
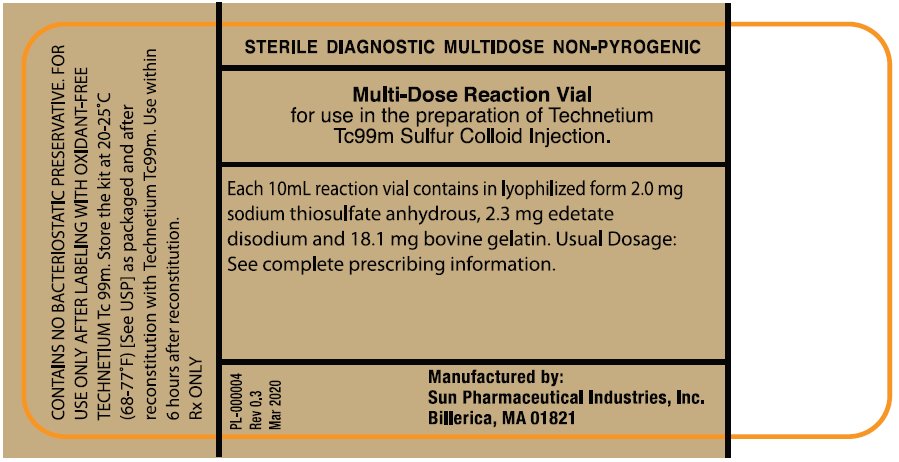
PRINCIPAL DISPLAY PANEL
NDC 045567-0030-1
STERILE DIAGNOSTIC MULTIDOSE NON-PYROGENIC
Multi-Dose Reaction Vial
for use in the Preparation of Technetium Tc 99m Sulfur Colloid Injection.
Each 10 mL reaction vial contains in lyophilized form 2.0 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg bovine gelatin. Usual Dosage: See complete prescribing information.
Manufactured by:
Sun Pharmaceutical Industries, Inc.
Billerica, MA 01821
PL-000004
Rev 0.3
Mar 2020
CONTAINS NO BACTERIOSTATIC PRESERVATIVE FOR
USE ONLY AFTER LABELING WITH OCIDANT-FREE
TECHENTIUM Tc 99m. Store the kit at 20-25°C
(68-77°F) [See USP] as packaged and after reconstitution with Technetium Tc 99m.
Use within 6 hours after reconstitution.
Rx ONLY
PRINCIPAL DISPLAY PANEL
NDC 045567-0030-1
A
Solution A vial contains 1.8mL sterile, non pyrogenic 0.148 N hydrochloric acid solution.
To be used only with the Sulfur Colloid Multi-dose Reaction Vial.
Single Use Vial-Discard Unused Portion
NOT FOR DIRECT INTRAVENOUS INJECTION.
RX ONLY. STORE AT 20-25°C (68-77°F) [See USP]
Manufactured By: Sun Pharmaceutical Industries, Inc. Billerica, MA 01821
PL-000002
Rev 0.2
Mar 2020

PRINCIPAL DISPLAY PANEL
NDC 045567-0030-1
B
Solution B vial contains 1.8mL sterile, non pyrogenic aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. To be used only with the Sulfur Colloid Multi-dose Reaction Vial.
Single Use Vial-Discard Unused Portion.
NOT FOR DIRECT INTRAVENOUS INJECTION.
RX ONLY. STORE AT 20-25°C (68-77°F) [See USP]
Manufactured By: Sun Pharmaceutical Industries, Inc. Billerica MA 01821
PL-000003
Rev 0.2
Mar 2020

PRINCIPAL DISPLAY PANEL
CAUTION RADIOACTIVE MATERIAL
STERILE, NON-PYROGENIC, DIAGNOSTIC MULTIDOSE TECHNETIUM Tc 99m SULFUR COLLOID
Subcutaneous, Intravenous, Oral, and Intraperitoneal Use
Total MBq (mCi)_____Volume_____
Assay_____MBq/mL(mCi/mL) as of _____
The 10 mL vial contents are made with 2 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium, 18.1 mg bovine gelatin, the added 1.5 mL of 0.148 N hydrochloric acid solution and the added 1.5 mL aqueous solution of 36.9 mg sodium biphosphate anhydrous and 11.9 mg sodium hydroxide. Contains no bacteriostatic preservative. For use only after labeling with oxidant-free Technetium Tc 99m. Store reconstituted vial at 20-25°C (68-77°F) [See USP]. Use within 6 hours after labeling with Technetium Tc 99m. Usual Dosage: See complete prescribing information. (SEE ENCLOSED PACKAGE INSERT) Rx only
Manufactured by:
Sun Pharmaceutical Industries, Inc. Billerica, MA 01821
PL-000005
Rev 0.2
Mar 2020

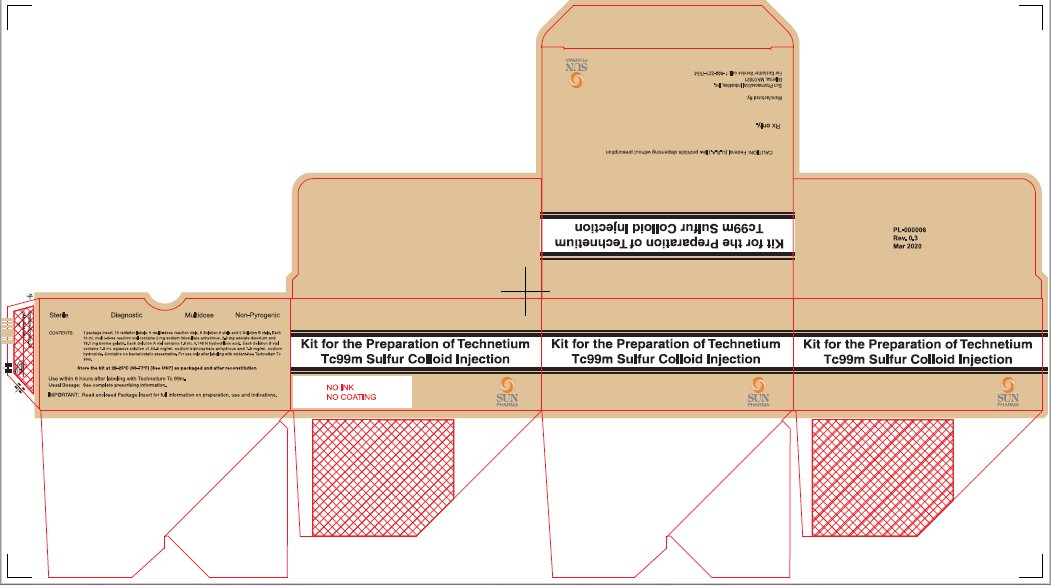
PRINCIPAL DISPLAY PANEL
NDC 045567-0030-1
Kit for the Preparation of Technetium Tc99m Sulfur Colloid Injection
CAUTION: Federal (U.S.A.) law prohibits dispensing without prescription
Rx only.
Manufactured By:
Sun Pharmaceutical Industries, Inc. Billerica, MA 01821
For Customer Service call: 1-800-221-7554
Sterile Diagnostic Multidose Non-Pyrogenic
CONTENTS: 1 package insert, 10 radiation labels, 5 multi-dose reaction vials, 5 Solution A vials and 5 Solution B vials. Each 10 mL multi-dose reaction vial contains 2 mg sodium thiosulfate anhydrous, 2.3 mg edetate disodium and 18.1 mg bovine gelatin. Each Solution A vial contains 1.8 mL 0.148 N hydrochloric acid. Each solution B vial contains 1.8 mL aqueous solution of 24.6 mg/mL sodium biphosphate anhydrous and 7.9 mg/mL sodium hydroxide. Contains no bacteriostatic preservative. For intravenous use only after labeling with oxidant-free Technetium Tc 99m.
Store the kit at 20-25°C (68-77°F) [See USP] as packaged and after reconstitution
Use within 6 hours after labeling with Technetium Tc 99m.
Usual Dosage: See complete prescribing information.
IMPORTANT: Read enclosed Package Insert for full information on preparation, use and indications.
PL-000006
Rev 0.3
Mar 2020