NDC Code(s) : 45802-580-84, 45802-580-46, 45802-580-62, 45802-580-01
Packager : Padagis Israel Pharmaceuticals Ltd
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Scopolamine Trandermal Systemscolopamine transdermal system PATCH, EXTENDED RELEASE | |||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Padagis Israel Pharmaceuticals Ltd(600093611) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aveva Drug Delivery Systems Inc. | 783982093 | analysis(45802-580), manufacture(45802-580) | |
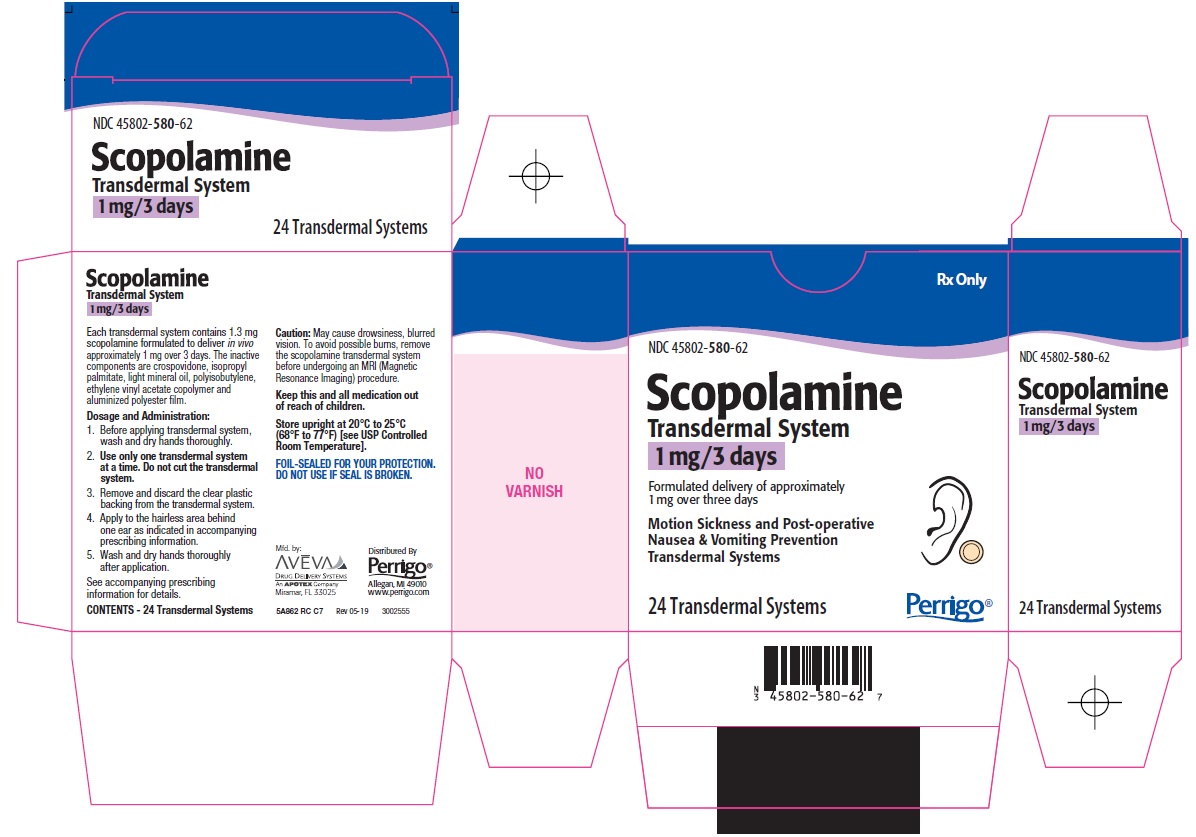
PRINCIPAL DISPLAY PANEL
Rx Only
Scopolamine Transdermal System
1 mg/3 days
Formulated delivery of approximately 1 mg over three days
Motion Sickness and Post-operative Nausea & Vomiting Prevention Transdermal Systems
24 Transdermal Systems
The following image is a placeholder representing the product identifier that is either affixed or imprinted on the drug package label during the packaging operation.










