NDC Code(s) : 45802-910-96, 45802-910-01
Packager : Perrigo New York Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Perrigo Benzoyl Peroxide Benzoyl Peroxide GEL | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Rx Only
Benzoyl Peroxide Gel, 5%
For Topical Use Only
Aqueous Base Acne Gel
 Benzoyl Peroxide Gel, 5% Carton
Benzoyl Peroxide Gel, 5% Carton
Benzoyl Peroxide Gel, 5% Carton
PRINCIPAL DISPLAY PANEL
Rx Only
Benzoyl Peroxide Gel, 5%
For Topical Use Only
Aqueous Base Acne Gel
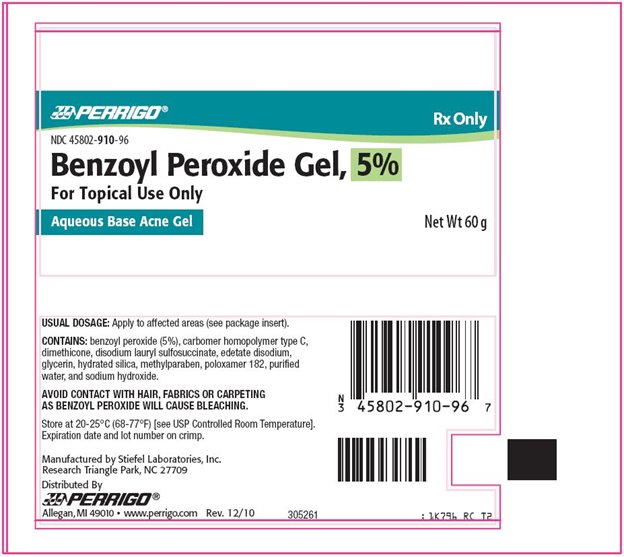 Benzoyl Peroxide Gel, 5% Tube
Benzoyl Peroxide Gel, 5% Tube
Benzoyl Peroxide Gel, 5% Tube









