NDC Code(s) : 47335-082-50, 47335-083-50
Packager : Sun Pharma Global FZE
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| LIPODOX Doxorubicin Hydrochloride INJECTABLE, LIPOSOMAL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LIPODOX 50 Doxorubicin Hydrochloride INJECTABLE, LIPOSOMAL | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
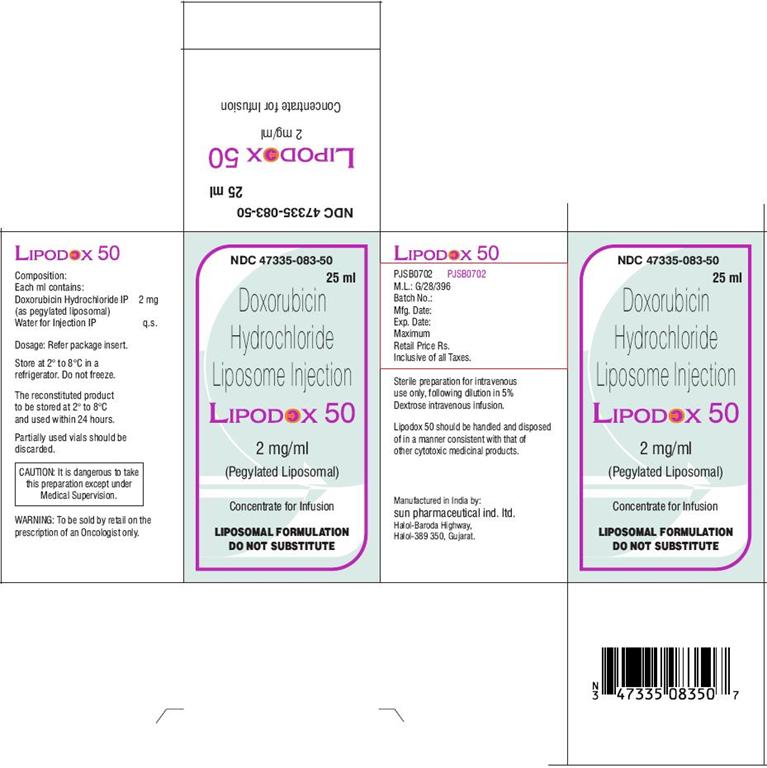
PRINCIPAL DISPLAY PANEL
NDC 47335-082-50
10 ml
Doxorubicin Hydrochloride Liposome Injection
LIPODOX
2 mg/ml
(Pegylated Liposomal)
Concentrate for Infusion
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE

PRINCIPAL DISPLAY PANEL
NDC 47335-083-50
25 ml
Doxorubicin Hydrochloride Liposome Injection
LIPODOX 50
2 mg/ml
(Pegylated Liposomal)
Concentrate for Infusion
LIPOSOMAL FORMULATION
DO NOT SUBSTITUTE