NDC Code(s) : 47593-406-41, 47593-406-80
Packager : Ecolab Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clean ForceEthyl Alcohol SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Ecolab Inc.(006154611) |
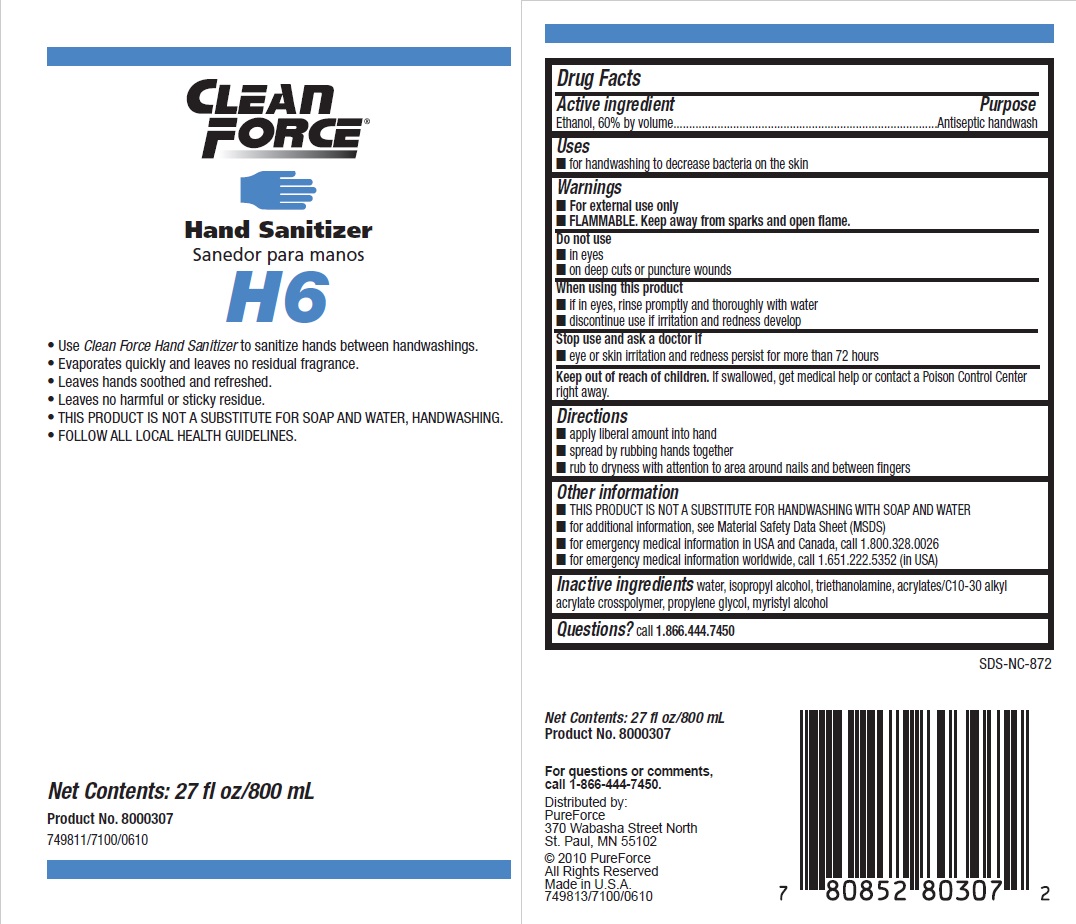
PRINCIPAL DISPLAY PANEL
CLEAN FORCE
HAND SANITIZER
Use Clean Force Hand Sanitizer to sanitize hands between handwashings.
Evaporates quickly and leaves no residual fragrance.
Leaves hands soothed and refreshed.
Leaves no harmful or sticky residue.
THIS PRODUCT IS NOT A SUBSTITUTE FOR SOAP AND WATER, HANDWASHING.
FOLLOW ALL LOCAL HEALTH GUIDELINES.
Net Contents: 27 fl oz/800 mL
Product No. 8000307749811/7100/0610
Distributed by:
PureForce
370 Wabasha Street North
St. Paul, MN 55102
© 2010 PureForce
All Rights Reserved
Made in U.S.A.
749813/7100/0610