NDC Code(s) : 47682-226-17
Packager : Unifirst First Aid Corporation
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Blood Clotting First Aidbenzethonium chloride and lidocaine AEROSOL, SPRAY | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| LABELER - Unifirst First Aid Corporation(832947092) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Dixon Investments, Inc. | 115315822 | manufacture(47682-226) | |
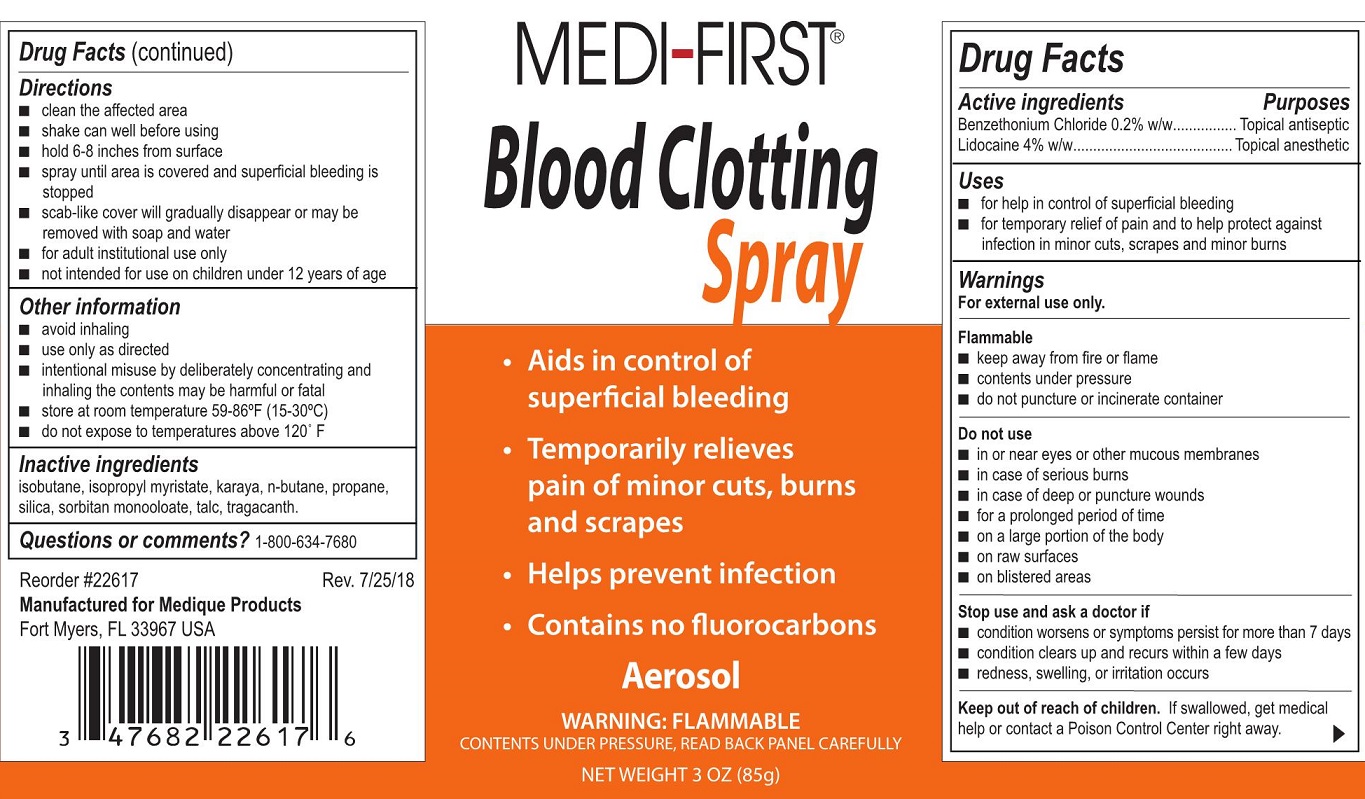
PRINCIPAL DISPLAY PANEL
Medi-First® Blood Clotting Spray
- Aids in control of bleeding
- Temporarily relieves pain of minor cuts, burns and scrapes
- Helps prevent infection
- Coantains no fluorocarbons
- Aerosol
- Warning: FLAMMABLE
- Contents under pressure, read Back Panel Carefully
- Net Weight 3 oz (85g)