NDC Code(s) : 49035-983-05, 49035-983-62
Packager : Wal-Mart Stores, Inc.
Category : Human OTC Drug Label
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Acid Reducer Omeprazole TABLET, DELAYED RELEASE | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - Wal-Mart Stores, Inc.(051957769) |
| REGISTRANT - Aurohealth LLC(078728447) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Aurobindo Pharma Limited | 650381903 | ANALYSIS(49035-983), MANUFACTURE(49035-983) | |
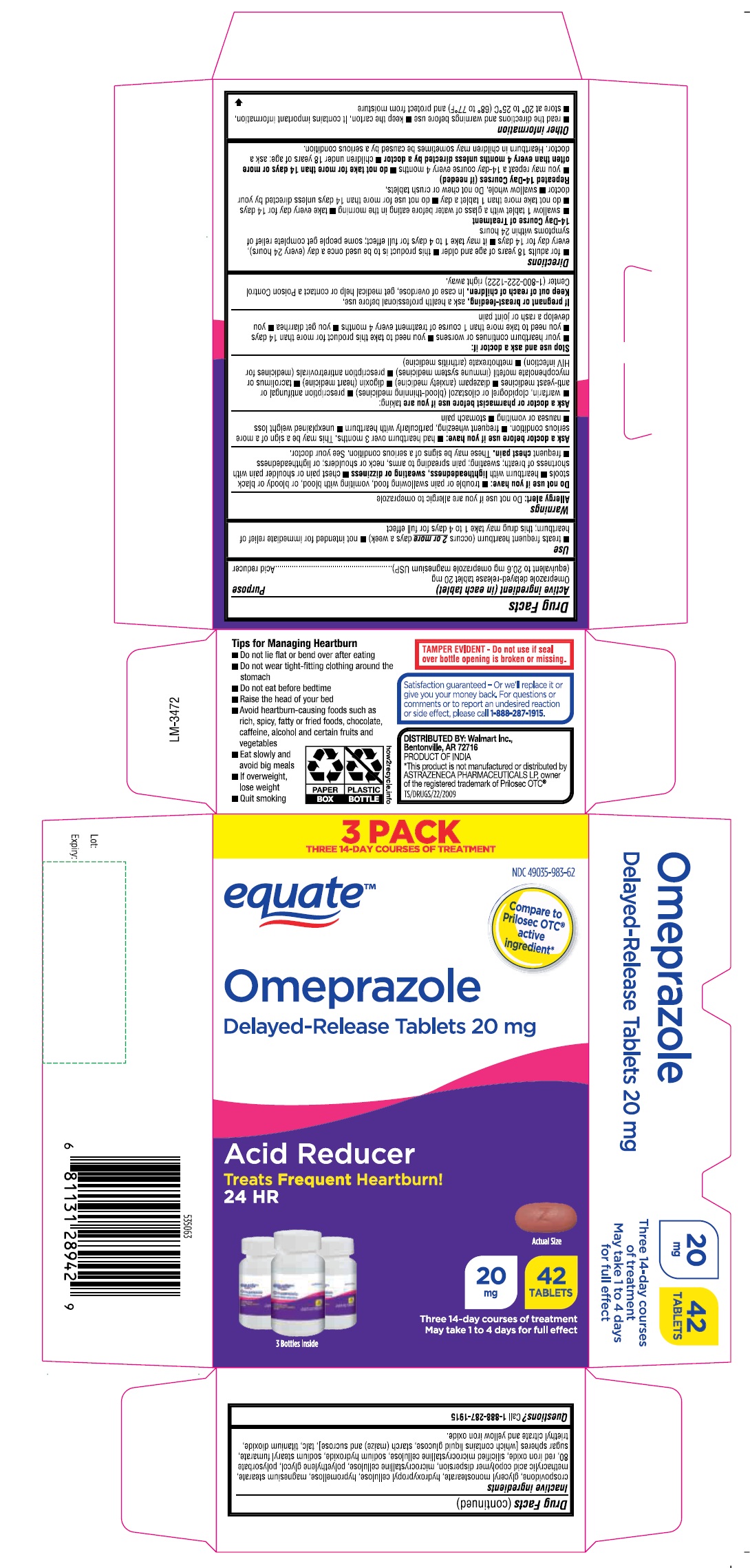
PRINCIPAL DISPLAY PANEL
equateTM
NDC 49035-983-05
Omeprazole
Delayed-Release
Tablets 20 mg
24 HR
ACID REDUCER
Treats Frequent Heartburn!
14 TABLETS
One 14-day course of treatment
•May take 1 to 4 days for full effect

PRINCIPAL DISPLAY PANEL
3 PACK
equateTM
NDC 49035-983-62
Compare to Prilosec OTC®
active ingredient*
Omeprazole
Delayed-Release
Tablets 20 mg
24 HR
ACID REDUCER
Treats Frequent Heartburn!
•May take 1 to 4 days for full effect
Actual Size
42
TABLETS
Three 14-day courses of treatment