NDC Code(s) : 49281-545-03
Packager : Sanofi Pasteur Inc.
Category : VACCINE LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| ActHIBHAEMOPHILUS INFLUENZAE TYPE B STRAIN 1482 CAPSULAR POLYSACCHARIDE TETANUS TOXOID CONJUGATE ANTIGEN KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LABELER - Sanofi Pasteur Inc.(086723285) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sanofi Pasteur SA | 578763542 | MANUFACTURE | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Sanofi Pasteur Inc. | 086723285 | MANUFACTURE | |
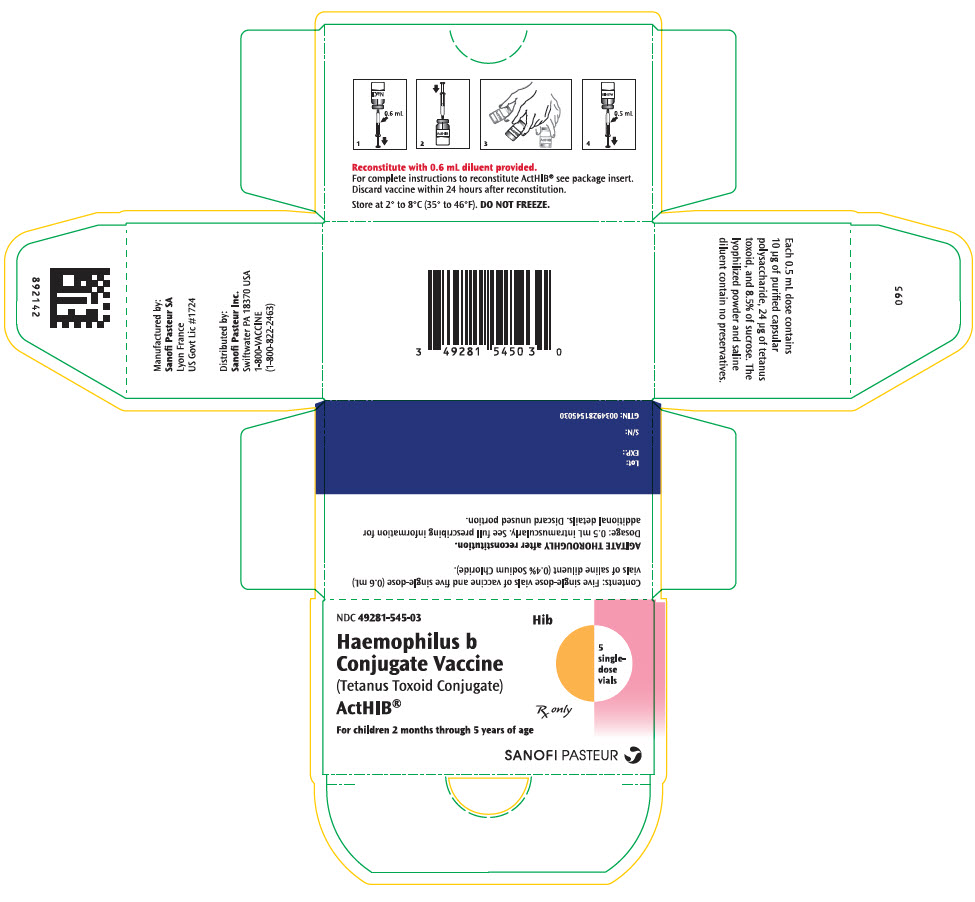
PRINCIPAL DISPLAY PANEL
NDC 49281-545-03
Hib
Haemophilus b
Conjugate Vaccine
(Tetanus Toxoid Conjugate)
ActHIB®
For children 2 months through 5 years of age
5
single-
dose
vials
Rx only
SANOFI PASTEUR
PRINCIPAL DISPLAY PANEL
NDC 49281-547-58
Hib
single-dose (0.5 mL)
Not to be used alone. 1 of 2
For use only after reconstitution
with accompanying Saline
Diluent Component.
Rx only
Haemophilus b Conjugate
Vaccine (Tetanus Toxoid
Conjugate)
ActHIB®
Mfd by: Sanofi Pasteur SA
892140
Lot
EXP

PRINCIPAL DISPLAY PANEL
NDC 49281-546-58
single-dose (0.6 mL)
Not to be used alone. 2 of 2
Saline Diluent Component
(0.4% Sodium Chloride)
For reconstitution of
ActHIB®
Haemophilus b Conjugate
Vaccine (Tetanus Toxoid
Conjugate)
Rx only
Mfd by: Sanofi Pasteur Inc.
Swiftwater PA 18370 USA
892139
Lot
EXP










