NDC Code(s) : 49281-820-10, 49281-800-83
Packager : Sanofi Pasteur Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TETANUS TOXOID ADSORBEDCLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| TETANUS TOXOID ADSORBEDCLOSTRIDIUM TETANI TOXOID ANTIGEN (FORMALDEHYDE INACTIVATED) INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
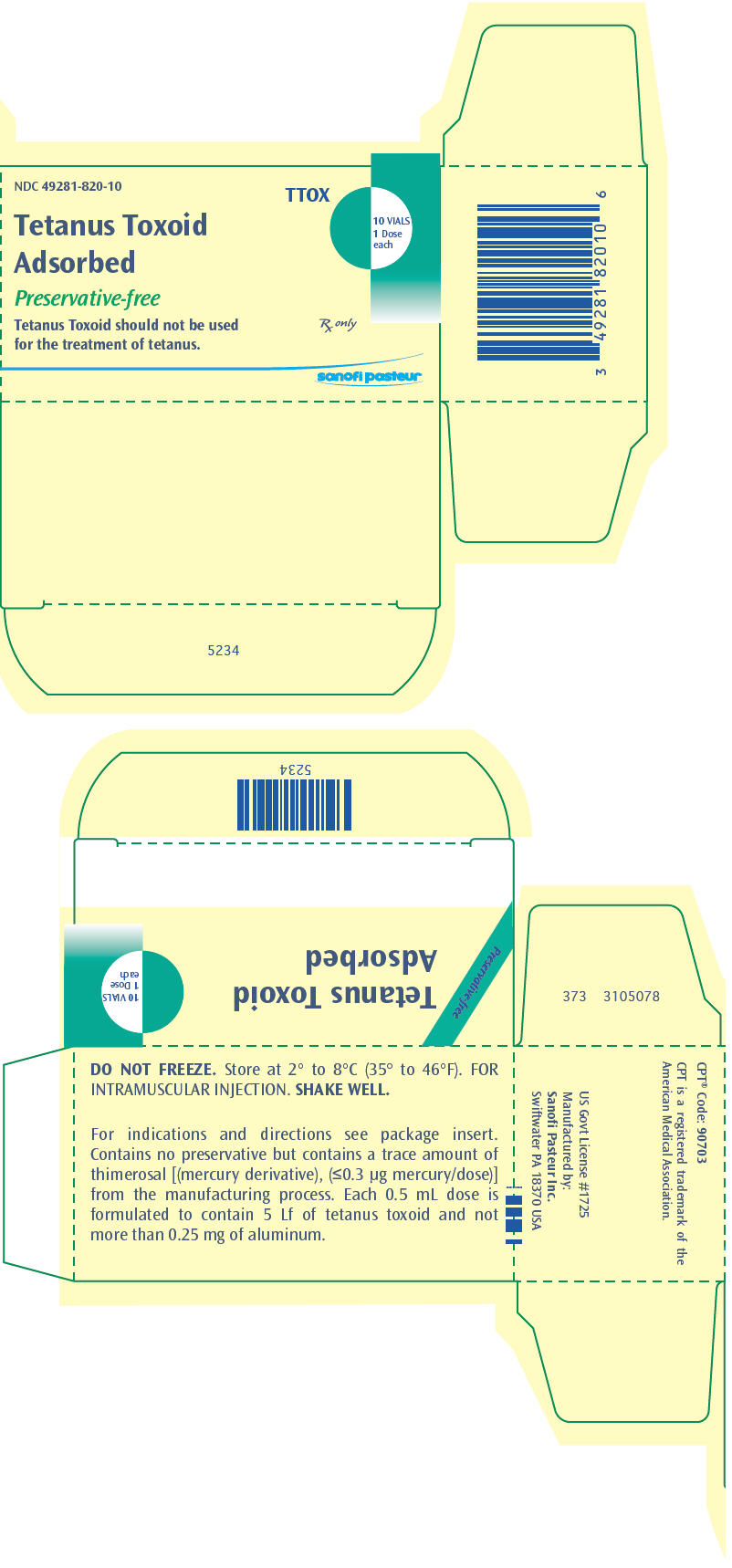
PRINCIPAL DISPLAY PANEL
NDC 49281-820-10
Tetanus
Toxoid
Adsorbed
TTOX
1 Dose
(0.5 mL)
Rx only
Preservative-free
Mfd by: Sanofi Pasteur Inc.

PRINCIPAL DISPLAY PANEL
NDC 49281-820-10
TTOX
Tetanus Toxoid
Adsorbed
10 VIALS
1 Dose
each
Preservative-free
Tetanus Toxoid should not be used
for the treatment of tetanus.
Rx only
sanofi pasteur