NDC Code(s) : 49401-102-01, 49401-101-01, 49401-088-42, 49401-088-47, 49401-088-50, 49401-088-01, 49401-088-35, 49401-088-02, 49401-088-61
Packager : GlaxoSmithKline LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| BENLYSTAbelimumab INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BENLYSTAbelimumab INJECTION, POWDER, LYOPHILIZED, FOR SOLUTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| BENLYSTAbelimumab SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - GlaxoSmithKline LLC(167380711) |
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
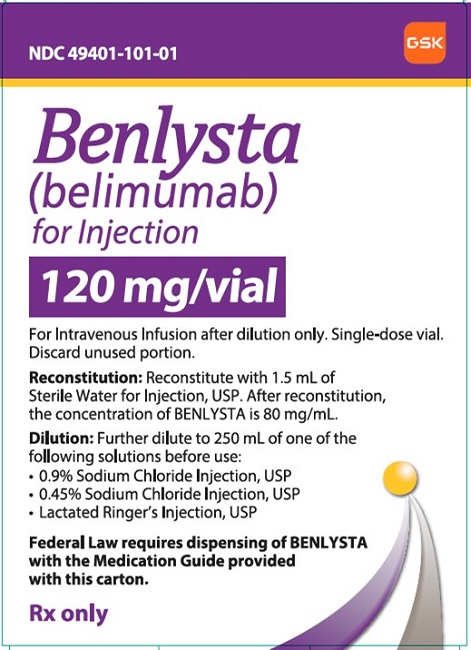
NDC 49401-101-01
Benlysta
(belimumab)
for Injection
120 mg/vial
GSK
For Intravenous Infusion after dilution only. Single-dose vial.
Discard unused portion
Reconstitution: Reconstitute with 1.5 mL of Sterile Water for Injection, USP. After reconstitution, the concentration of BENLYSTA is 80 mg/mL.
Dilution: Further dilute to 250 mL of one of the following solutions before use:
- •0.9% Sodium Chloride Injection, USP
- •0.45% Sodium Chloride Injection, USP
- •Lactated Ringer’s Injection, USP
Federal Law requires dispensing of BENLYSTA with the Medication Guide provided with this carton.
Rx only
©2022 GSK group of companies or its licensor.
Rev. 9/22
62000000082255
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 49401-102-01
Benlysta
(belimumab)
for Injection
400 mg/vial
GSK
For Intravenous Infusion after dilution only. Single-dose vial.
Discard unused portion.
Reconstitution: Reconstitute with 4.8 mL of Sterile Water for Injection, USP. After reconstitution, the concentration of BENLYSTA is 80 mg/mL.
Dilution: Further dilute to 250 mL of one of the following solutions before use:
- •0.9% Sodium Chloride Injection, USP
- •0.45% Sodium Chloride Injection, USP
- •Lactated Ringer’s Injection, USP
Federal Law requires dispensing of BENLYSTA with the Medication Guide provided with this carton.
Rx only
©2022 GSK group of companies or its licensor.
Rev. 9/22
62000000082242

PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL
NDC 49401-088-35
Benlysta
(belimumab)
Injection
200 mg/mL
Rx only
GSK
Once-weekly
For Subcutaneous Use
Contents:
- • 4 Single-Dose 1-mL Prefilled Autoinjectors
- • Instructions for Use (Read carefully)
- • Medication Guide
- • Prescribing Information
Federal Law requires dispensing of BENLYSTA with the Medication Guide provided with this package.
©2023 GSK group of companies or is licensor.
Rev. 4/23
62000000087694










