NDC Code(s) : 49836-740-01
Packager : RX PHARMA-PACK, INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| MLP A-1Bupivacaine Hydrochloride, Lidocaine Hydrochloride, Triamcinolone Acetonide, and Povidone-Iodine KIT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
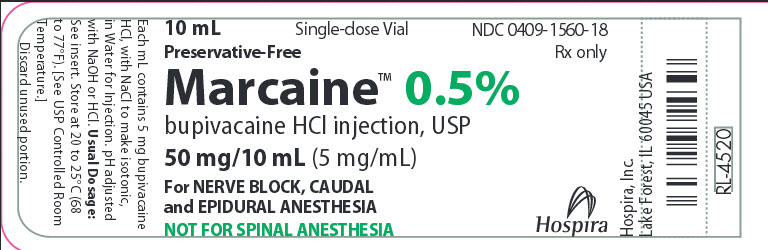
10 mL Single-dose Vial NDC 0409-1560-18
Preservative-Free Rx only
Marcaine™ 0.5%
bupivacaine HCl injection, USP
50mg/10mL (5mg/mL)
For NERVE BLOCK,CAUDAL
and EPIDURAL ANESTHESIA
NOT FOR SPINAL ANESTHESIA
Hospira
Each mL contains 5 mg bupivacaine
HCI, with NaCl to make isotonic,
in Water for Injection. pH adjusted
with NaOH or HCl. Usual Dosage:
See insert. Store at 20 to 25ºC (68
to 77ºF). [See USP Controlled Room
Temperature.]
Discard unused portion.
Hospira, Inc.
Lake Forest, IL 60045 USA
 Marcaine Image
Marcaine Image
PRINCIPAL DISPLAY PANEL
NDC 0409-4282-01
Rx only
2 mL Single-dose
Preservative-Free
2% LIDOCAINE HCl
Injection, USP
20 mg/mL
RL-0866 (11/04)
HOSPIRA, INC,
LAKE FOREST, IL
60045 USA

PRINCIPAL DISPLAY PANEL
NDC 0003-0293-05
KENALOG®-40
(Triamcinolone
Acetonide Injectable
Suspension, USP)
40 mg per 1 mL
Rx only
Read all side
Bristol-Myers Squibb
Each mL provides 40 mg
triamcinolone acetonide with
0.65% sodium chloride for
isotonicity, 0.99% (w/v) benzyl
alcohol added as a preservative,
0.75% carboxymethylcellulose
sodium, and 0.04% polysorbate
80 in an aqueous suspension;
sodium hydroxide or hydrochloric
acid may be present to adjust
pH to 5.0-7.5.
Shake well before using.
For Intramuscular or
intra-articular use only.
NOT FOR IV, ID, INTRAOCULAR,
EPIDURAL, OR INTRATHECAL
USE.
See insert for dosage
information.
Bristol-Myers Squibb Company
Princeton, NJ 08543 USA
Product of Spain 1132705A5
Store at controlled room
temperature, 20º -25ºC
(68º-77ºF), avoid freezing and
protect from light.
Do not refrigerate.
Store vial in carton.
See bottom panel for lot number
and expiration date.
 Kenalog-40
Kenalog-40
PRINCIPAL DISPLAY PANEL
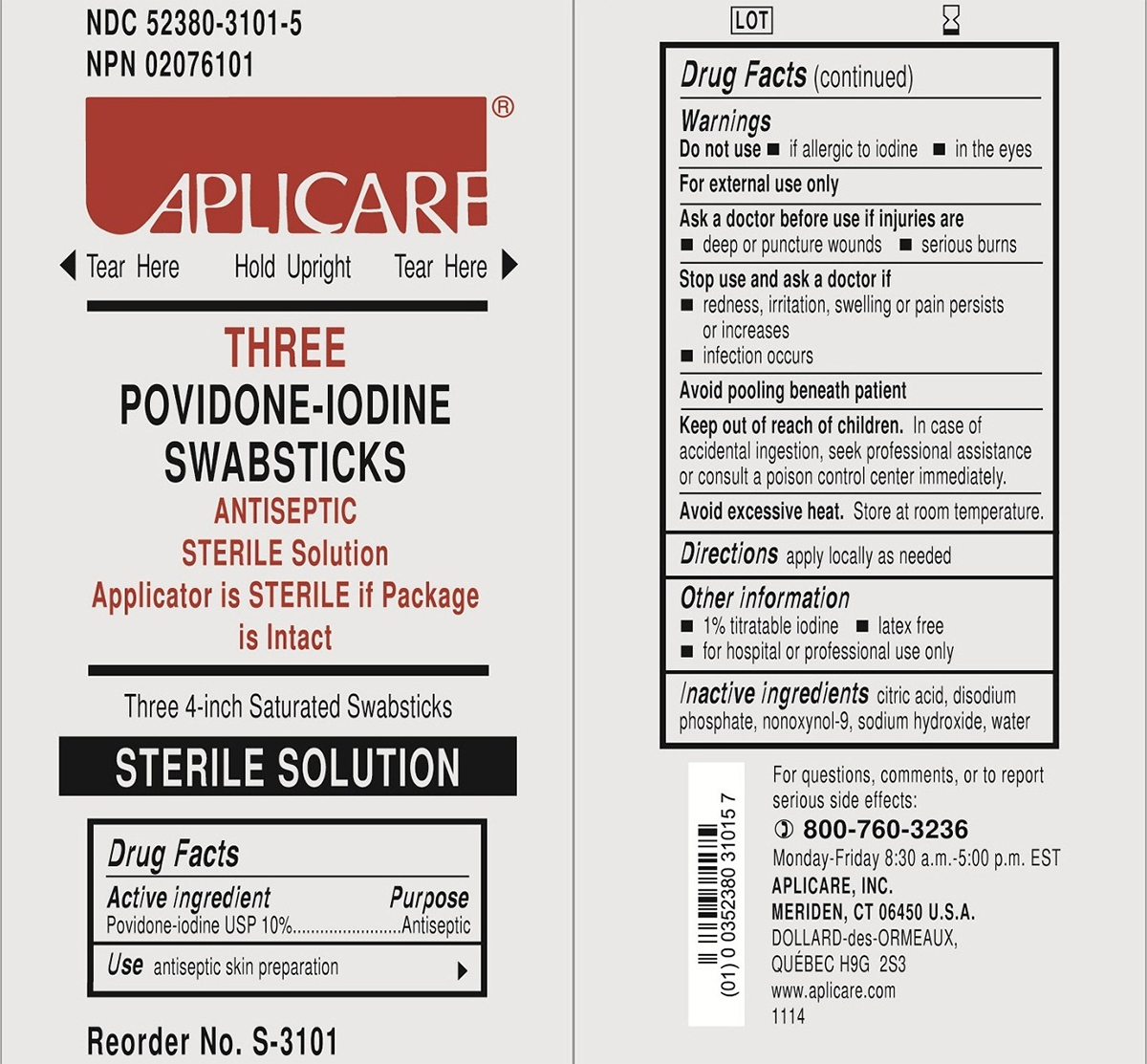
NDC 52380-3101-5
NPN 02076101
APLICARE®
Tear Here Hold Upright Tear Here
THREE
POVIDONE-IODINE
SWABSTICKS
ANTISEPTIC
STERILE Solution
Applicator is STERILE if Package
is Intact
Three 4-inch Saturated Swabsticks
STERILE SOLUTION
Reorder No. S-3101
For questions, comments, or to report
serious side effects:
800-760-3236
Monday-Friday 8:30 a.m.-5:00 p.m. EST
APLICARE, INC.
MERIDEN, CT 06450 U.S.A.
DOLLARD-des-ORMEAUX,
QUEBEC H9G 2S3
www.aplicare.com
1114
 Povidone Iodine
Povidone Iodine
PRINCIPAL DISPLAY PANEL
NDC 49836-740-01
RX-Only
MLP A-1
Kit Contains:
2 Kenalog-40® Single Dose Vial (1mL)
1 Marcaine® 0.5% Single Dose Vial (5mg/mL) (10mL)
1 Lidocaine HCl Injection, USP 2% Single Dose Ampule (20mg/mL) (2mL)
1 Sterile Povidone-Iodine Swabsticks (3 Swabs)
1 Pair Nitrile Powder Free Sterile Gloves (Size 7.5)
1 Sterile Towel Drape
1 Fenestrated Sterile Towel Drape
1 Sterile Adhesive Bandage
1 Pack of Sterile 4x4 Gauze (2 Gauzes)
1 Dose
Needles and Syringes Not Included
Distributed by:
DANEX INTERNATIONAL LLC
SPRING, TXl 77388
MANUFACTURED BY:
Rx
Pharma
Pack
HAUPPAUGE, NY 11788
Questions/Comments 1-844-632-7898
Kit Contents:
Kenalog-40® † (Bristol-Myers Squibb)*
Triamcinolone Acetonide Injectable Suspension, USP
Each mL Provides 40 mg triamcinolone acetonide with 0.65% sodium chloride for isotonicity, 0.99% (w/v) benzyl alcohol added as a preservative, 0.75% carboxymethylcellulose sodium, and 0.04% polysorbate 80 in an aqueous suspension; sodium hydroxide or hydrochloric acid may be present to adjust pH to 5.0-7.5.
Marcaine® ‡ 0.5% (5mg/mL) (Hospira, Inc.)*
Each mL contains 5mg bupivacaine HCl, with NaCl to make isotonic, in Water for Injection. pH adjusted with NaOH or HCl.
Lidocaine HCl Injection, USP 2% (20mg/mL) (Hospira, Inc.)*
Each mL contains lidocaine hydrochloride, anhydrous 20mg; sodium chloride 6mg. May contain sodium hydroxide and/or hydrochloric acid for pH adjustment. pH 6.5 (5.0 to 7.0). 0.31 mOsmol/mL (calc).
Sterile Povidone-lodine Swabsticks (Aplicare)*
Nitrile Powder Free Sterile Gloves (Dynarex) Size 7.5*
Sterile Towel Drape (Dynarex)*
Fenestrated Sterile Towel Drape (Dynarex)*
Sterile Adhesive Bandage (Dynarex)*
Sterile 4x4 Gauze (Dynarex)*
† Kenalog® (registered trademark of Bristol-Myers Squibb)
‡ Marcaine® (registered trademark of Hospira, Inc.)
* Internal package components remain sterile as long as items are unopened and undamaged
WARNING: KEEP THIS AND ALL MEDICATION OUT
OF THE REACH OF CHILDREN. IN CASE OF
ACCIDENTAL OVERDOSE, SEEK PROFESSIONAL
ASSISTANCE OR CONTACT A POISON CONTROL
CENTER IMMEDIATELY.
PROTECT FROM LIGHT I AVOID FREEZING
STORE AT CONTROLLED ROOM TEMPERATURE
20º-25ºC (68º-77º F) [SEE USP CONTROLLED
ROOM TEMPERATURE]
DO NOT REFRIGERATE.
Directions for Use: See enclosed inserts.
 MLP A-1
MLP A-1









