NDC Code(s) : 49967-150-01, 49967-150-02, 49967-150-03
Packager : L'Oreal USA Products Inc
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
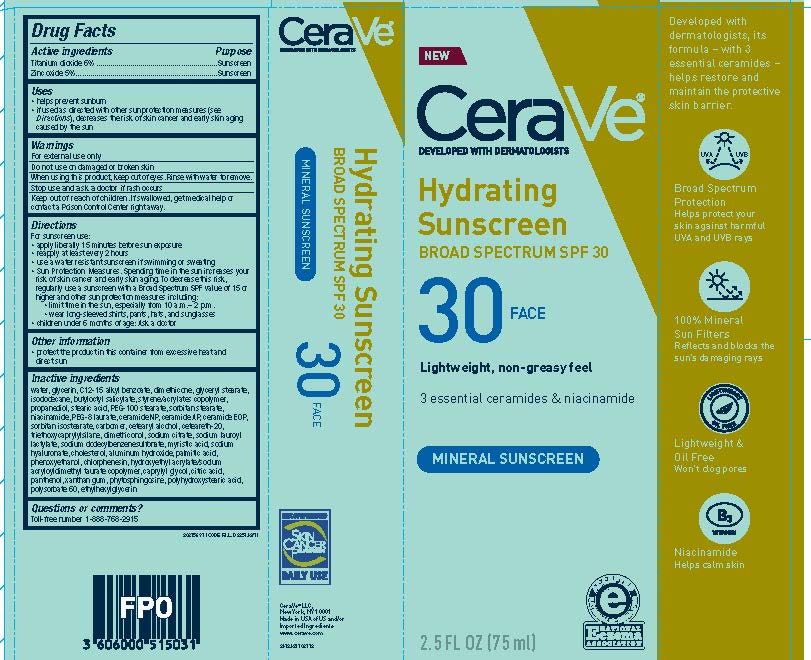
| CERAVE DEVELOPED WITH DERMATOLOGISTS HYDRATING SUNSCREEN SPF 30 FACE LIGHTWEIGHT, NON-GREASY FEEL 3 ESSENTIAL CERAMIDES NIACINAMIDE MINERAL SUNSCREENTitanium dioxide and zinc oxide LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - L'Oreal USA Products Inc(002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| L'OREAL USA PRODUCTS, INC. | 624244349 | manufacture(49967-150) | |
PRINCIPAL DISPLAY PANEL