NDC Code(s) : 49967-512-01, 49967-512-02, 49967-512-03
Packager : L'Oreal USA Products Inc
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CeraVe Developed with Dermatologists Itch Relief Moisturizingpramoxine hydrochloride CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - L'Oreal USA Products Inc(002136794) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Accupac, Inc. | 071609663 | MANUFACTURE(49967-512) | |
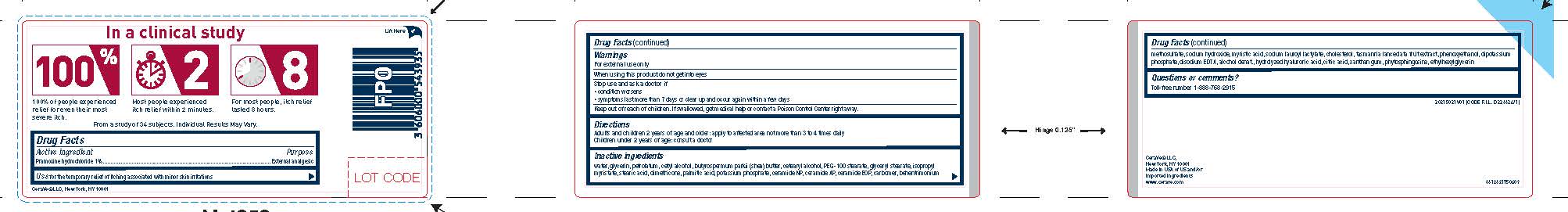
PRINCIPAL DISPLAY PANEL


PRINCIPAL DISPLAY PANEL