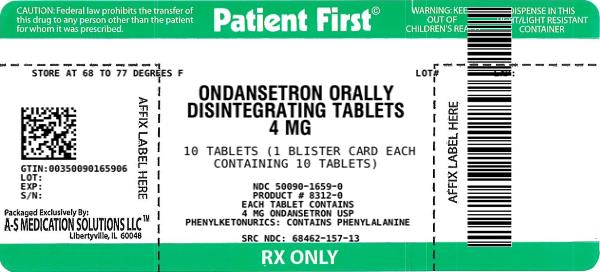
NDC Code(s) : 50090-1659-0
Packager : A-S Medication Solutions
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| OndansetronOndansetron TABLET, ORALLY DISINTEGRATING | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - A-S Medication Solutions(830016429) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| A-S Medication Solutions | 830016429 | RELABEL(50090-1659), REPACK(50090-1659) | |
PRINCIPAL DISPLAY PANEL