NDC Code(s) : 50458-657-30, 50458-655-30, 50458-653-30
Packager : Janssen Pharmaceuticals, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : CIV
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ultram ERTramadol Hydrochloride TABLET, EXTENDED RELEASE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Ultram ERTramadol Hydrochloride TABLET, EXTENDED RELEASE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Ultram ERTramadol Hydrochloride TABLET, EXTENDED RELEASE | ||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
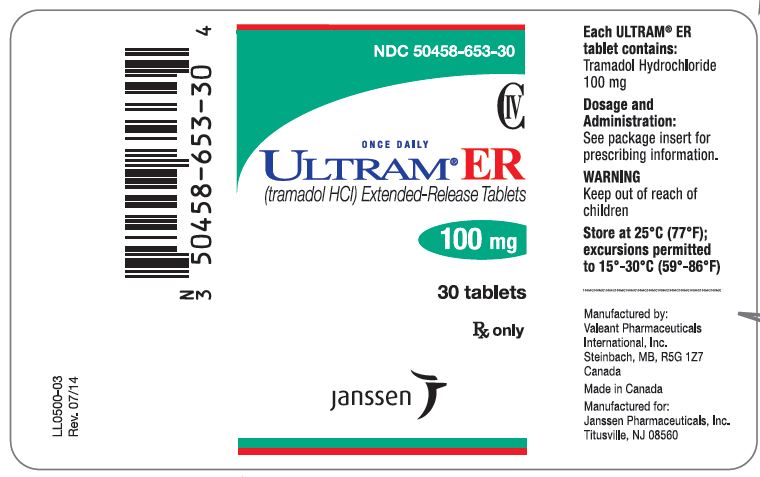
PRINCIPAL DISPLAY PANEL
NDC 50458-657-30
CIV
ONCE DAILY
ULTRAM® ER
(tramadol HCl) Extended-Release Tablets
300 mg
30 tablets
Rx only
janssen
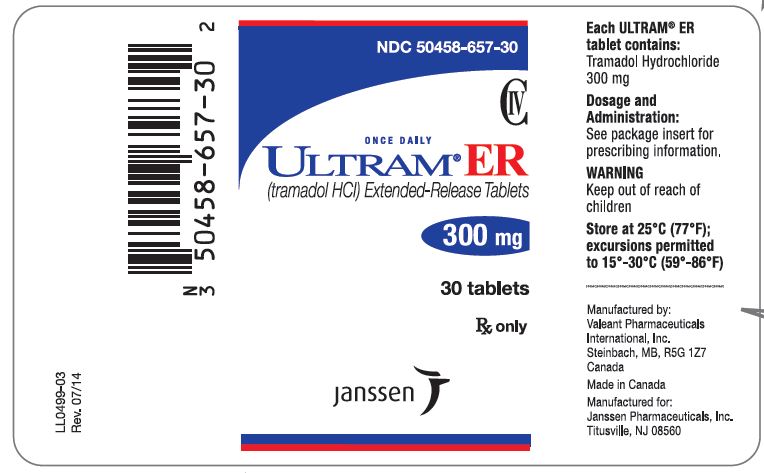
PRINCIPAL DISPLAY PANEL
NDC 50458-655-30
CIV
ONCE DAILY
ULTRAM® ER
(tramadol HCl) Extended-Release Tablets
200 mg
30 tablets
Rx only
janssen
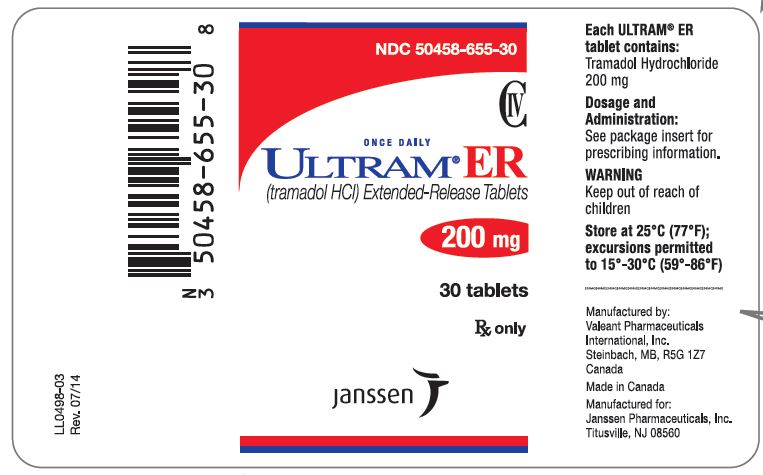
PRINCIPAL DISPLAY PANEL
NDC 50458-653-30
CIV
ONCE DAILY
ULTRAM® ER
(tramadol HCl) Extended-Release Tablets
100 mg
30 tablets
Rx only
Janssen