NDC Code(s) : 50544-100-11, 50544-100-94, 50544-101-10, 50544-101-94
Packager : University Medical Pharmaceuticals Corp
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
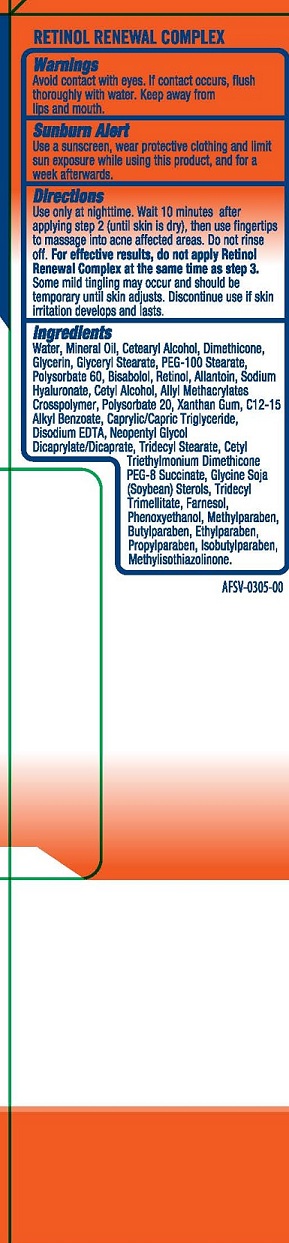
INGREDIENTS AND APPEARANCE
| AcneFree Severe Antibacterial Cleansing Wash Benzoyl Peroxide CREAM | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| AcneFree Severe Maximum Strength Repair Lotion Benzoyl Peroxide LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Severe to Clear Skin System
The ONLY TIME-RELEASED 10% Benzoyl Peroxide and Retinol System
The Most Powerful Acne-Healing Medicine Available Without A Prescription
Visible Clearing in Just Days Plus 24/7 Breakout Control
Medicine Stays in Pores to Continuously Fight Each Stage of Severe Acne
Dermatologist Tested - For All Ages and Skin Types








The ONLY TIME-RELEASED 10% Benzoyl Peroxide and Retinol System
The Most Powerful Acne-Healing Medicine Available Without A Prescription
Visible Clearing in Just Days Plus 24/7 Breakout Control
Medicine Stays in Pores to Continuously Fight Each Stage of Severe Acne
Dermatologist Tested - For All Ages and Skin Types


















