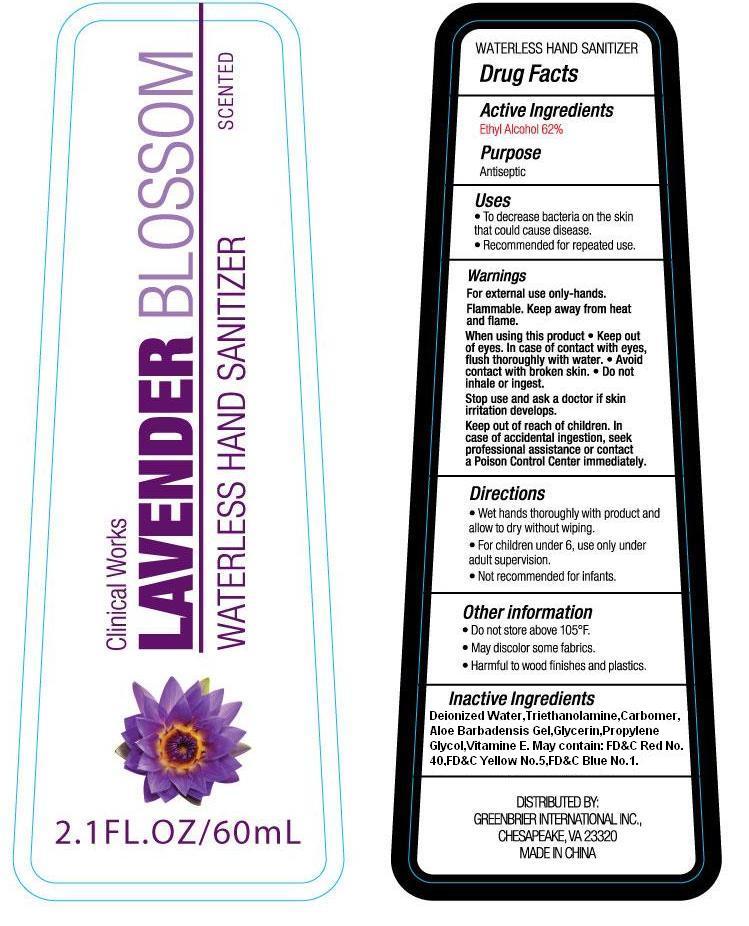
NDC Code(s) : 50593-006-01
Packager : Taizhou Xinzhixuan Daily-Use Co., Ltd.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Clinical Works Lavender Blossom Waterless Hand Sanitizer Alcohol LIQUID | ||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| LABELER - Taizhou Xinzhixuan Daily-Use Co., Ltd.(420438920) |
| REGISTRANT - Taizhou Xinzhixuan Daily-Use Co., Ltd.(420438920) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Taizhou Xinzhixuan Daily-Use Co., Ltd. | 420438920 | manufacture | |
PRINCIPAL DISPLAY PANEL
labe