NDC Code(s) : 50718-0012-1
Packager : Kamedis
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Calm Eczema Therapy WashSulphur 12X LIQUID | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| LABELER - Kamedis(080311300) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Biogenesis Inc. | 069117328 | manufacture(50718-0012) | |
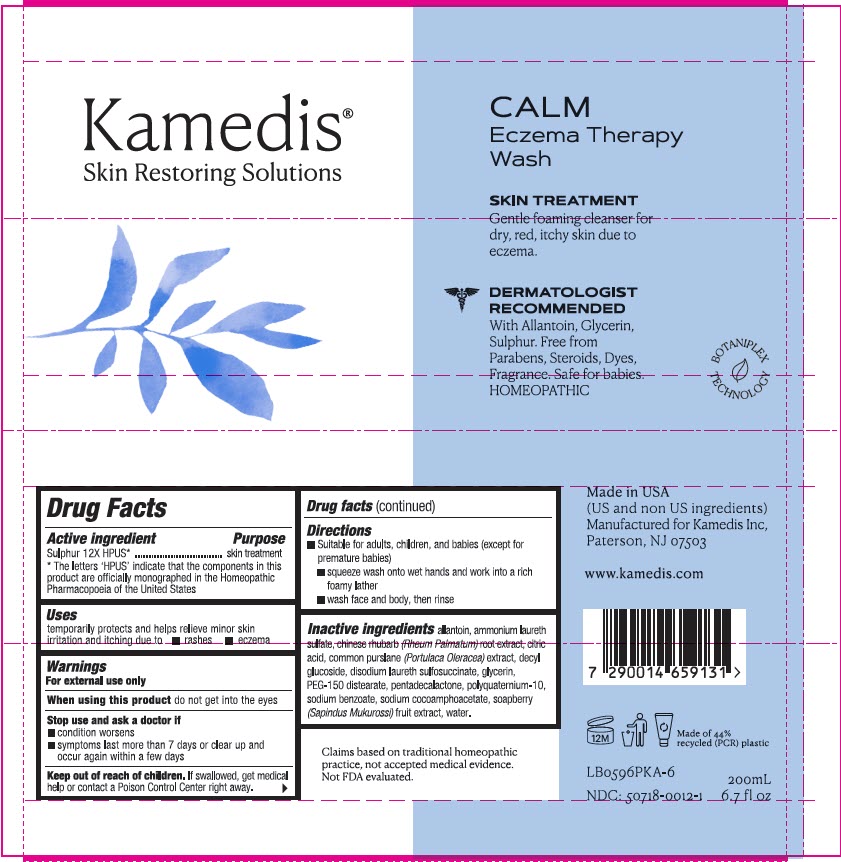
PRINCIPAL DISPLAY PANEL
Kamedis®
Skin Restoring Solutions
CALM
Eczema Therapy
Wash
SKIN TREATMENT
Gentle foaming cleanser for
dry, red, itchy skin due to
eczema.
DERMATOLOGIST
RECOMMENDED
With Allantoin, Glycerin,
Sulphur. Free from
Parabens, Steroids, Dyes,
Fragrance. Safe for babies.
HOMEOPATHIC
BOTANIPLEX
TECHNOLOGY