NDC Code(s) : 50718-0021-1, 50718-0021-2
Packager : Kamedis
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Control Dandruff Therapydandruff therapy shampoo SHAMPOO | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| LABELER - Kamedis(080311300) |
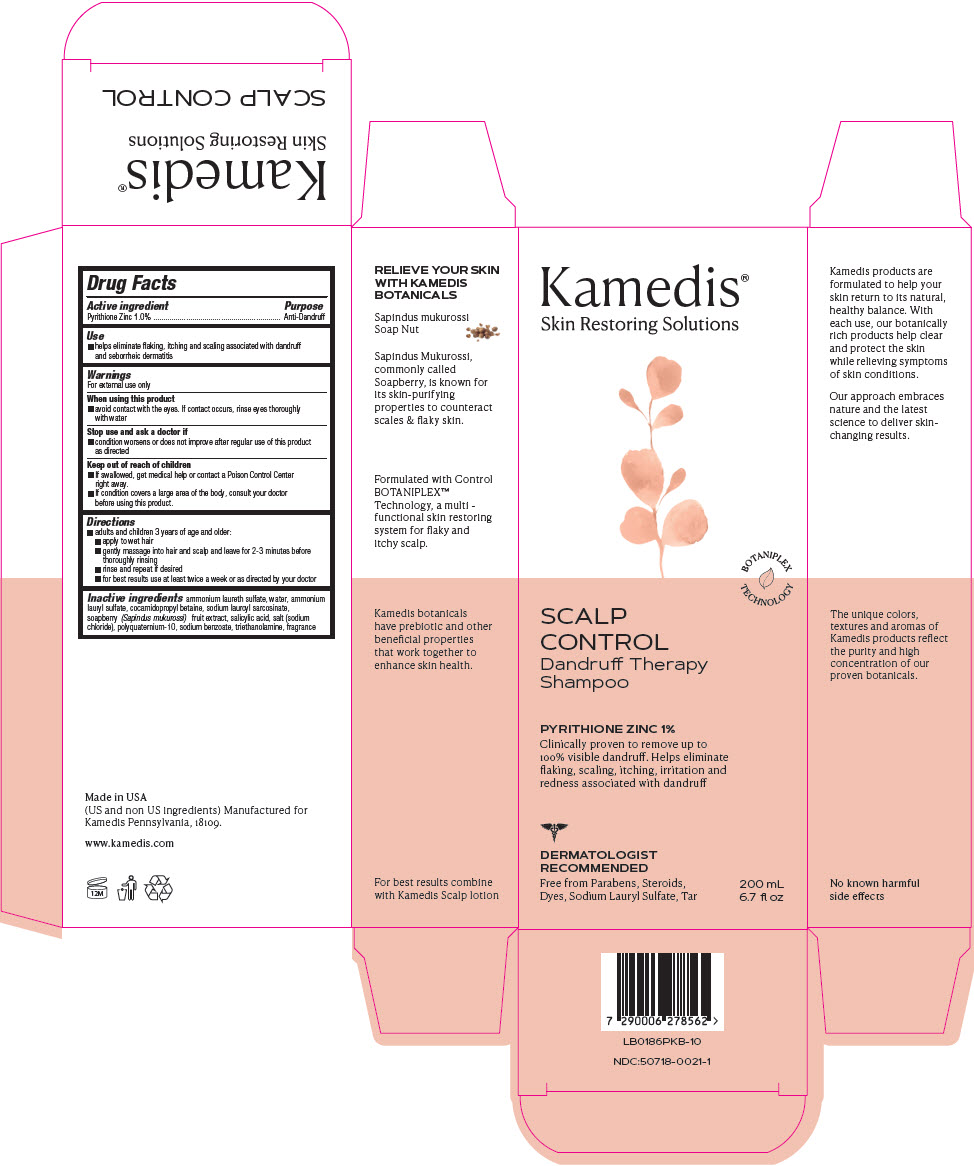
PRINCIPAL DISPLAY PANEL
Kamedis®
Skin Restoring Solutions
BOTANIPLEX
TECHNOLOGY
SCALP
CONTROL
Dandruff Therapy
Shampoo
PYRITHIONE ZINC 1%
Clinically proven to remove up to
100% visible dandruff. Helps eliminate
flaking, scaling, itching, irritation and
redness associated with dandruff
DERMATOLOGIST
RECOMMENDED
Free from Parabens, Steroids,
Dyes, Sodium Lauryl Sulfate, Tar
200 mL
6.7 fl oz