NDC Code(s) : 51013-190-30, 51013-190-09
Packager : PuraCap Pharmaceutical LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| DutasterideDutasteride CAPSULE, LIQUID FILLED | ||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||
| LABELER - PuraCap Pharmaceutical LLC(962106329) |
| REGISTRANT - Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd(421293287) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Humanwell Puracap Pharmaceuticals (Wuhan) Co., Ltd | 421293287 | MANUFACTURE(51013-190) | |
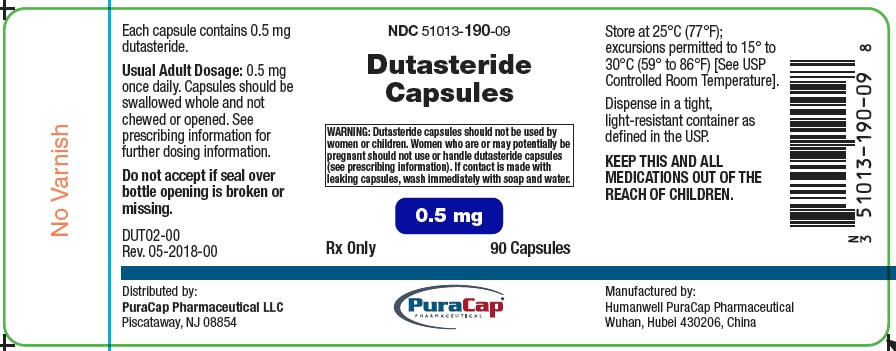
PRINCIPAL DISPLAY PANEL
Dutasteride Capsules 0.5 mg
WARNING: Dutasteride capsules should not be used by women or children. Women who are or may potentially be pregnant should not use or handle dutasteride capsules (see prescribing information). If contact is made with leaking capsules, wash immediately with soap and water.
Rx Only