NDC Code(s) : 51060-063-01, 51060-064-01, 51060-065-01, 51060-066-01, 51060-067-01
Packager : Tarte, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Amazonian Colored Clay Foundation Broad Spectrum SPF 15 Sunscreen Titanium Dioxide and Zinc Oxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amazonian Colored Clay Foundation Broad Spectrum SPF 15 Sunscreen Titanium Dioxide and Zinc Oxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amazonian Colored Clay Foundation Broad Spectrum SPF 15 Sunscreen Titanium Dioxide and Zinc Oxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amazonian Colored Clay Foundation Broad Spectrum SPF 15 Sunscreen Titanium Dioxide and Zinc Oxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Amazonian Colored Clay Foundation Broad Spectrum SPF 15 Sunscreen Titanium Dioxide and Zinc Oxide LIQUID | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
tarte
high-performance naturals™
CC
Amazonian colored clay liquid foundation
Broad Spectrum
SPF 15 Sunscreen

PRINCIPAL DISPLAY PANEL
tarte
high-performance naturals™
CC
Amazonian colored clay liquid foundation
Broad Spectrum
SPF 15 Sunscreen

PRINCIPAL DISPLAY PANEL
tarte
high-performance naturals™
CC
Amazonian colored clay liquid foundation
Broad Spectrum
SPF 15 Sunscreen
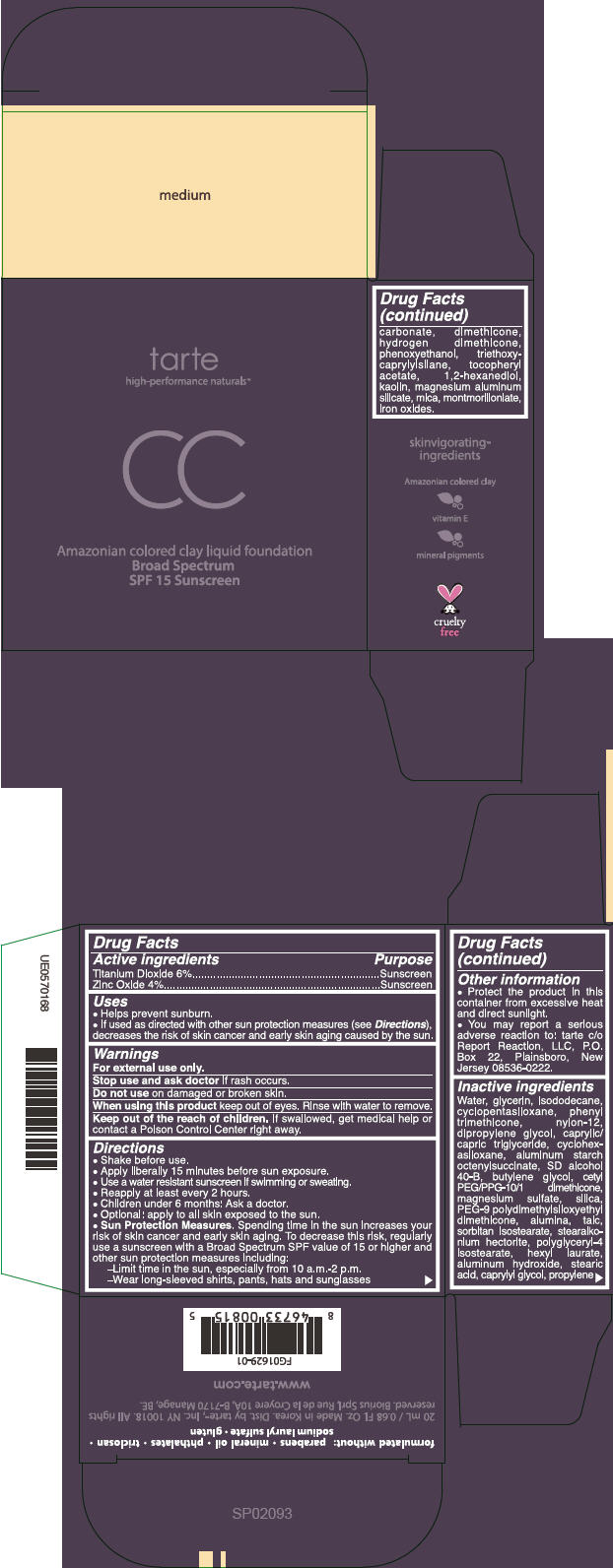
PRINCIPAL DISPLAY PANEL
tarte
high-performance naturals™
CC
Amazonian colored clay liquid foundation
Broad Spectrum
SPF 15 Sunscreen

PRINCIPAL DISPLAY PANEL
tarte
high-performance naturals™
CC
Amazonian colored clay liquid foundation
Broad Spectrum
SPF 15 Sunscreen










