NDC Code(s) : 51079-192-01, 51079-192-03, 51079-193-01, 51079-193-03, 51079-194-01, 51079-194-03
Packager : Mylan Institutional Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Valsartan and Hydrochlorothiazidevalsartan and hydrochlorothiazide TABLET, FILM COATED | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Valsartan and Hydrochlorothiazidevalsartan and hydrochlorothiazide TABLET, FILM COATED | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| Valsartan and Hydrochlorothiazidevalsartan and hydrochlorothiazide TABLET, FILM COATED | ||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
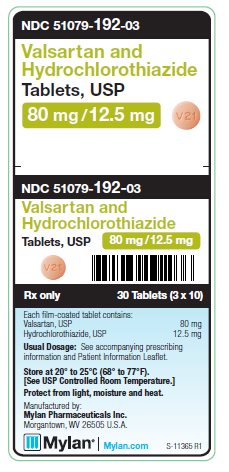
PRINCIPAL DISPLAY PANEL - 80 mg/ 12.5 mg
NDC 51079-192-03
Valsartan and
Hydrochlorothiazide
Tablets, USP
80 mg/12.5 mg
30 Tablets (3 x 10)
Each film-coated tablet contains:
Valsartan, USP 80 mg
Hydrochlorothiazide, USP 12.5 mg
Usual Dosage: See accompanying
prescribing information and
Patient Information Leaflet.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light, moisture and heat.
Manufactured by:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Rx only
S-11365 R1
Packaged and Distributed by:
Mylan Institutional Inc.
Rockford, IL 61103 U.S.A.
This unit dose package is not child resistant.
For institutional use only.
Keep this and all drugs out of the reach of children.
This container provides light-resistance.
See window for lot number and expiration date.
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 160 mg/ 12.5 mg
NDC 51079-193-03
Valsartan and
Hydrochlorothiazide
Tablets, USP
160 mg/12.5 mg
30 Tablets (3 x 10)
Each film-coated tablet contains:
Valsartan, USP 160 mg
Hydrochlorothiazide, USP 12.5 mg
Usual Dosage: See accompanying
prescribing information and
Patient Information Leaflet.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light, moisture and heat.
Manufactured by:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Rx only
S-11366 R1
Packaged and Distributed by:
Mylan Institutional Inc.
Rockford, IL 61103 U.S.A.
This unit dose package is not child resistant.
For institutional use only.
Keep this and all drugs out of the reach of children.
This container provides light-resistance.
See window for lot number and expiration date.

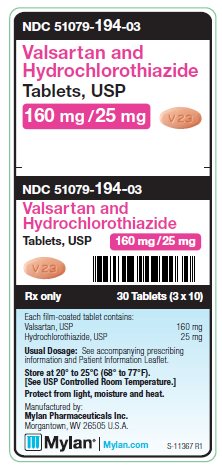
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 160 mg/ 25 mg
NDC 51079-194-03
Valsartan and
Hydrochlorothiazide
Tablets, USP
160 mg/25 mg
30 Tablets (3 x 10)
Each film-coated tablet contains:
Valsartan, USP 160 mg
Hydrochlorothiazide, USP 25 mg
Usual Dosage: See accompanying
prescribing information and
Patient Information Leaflet.
Store at 20° to 25°C (68° to 77°F).
[See USP Controlled Room Temperature.]
Protect from light, moisture and heat.
Manufactured by:
Mylan Pharmaceuticals Inc.
Morgantown, WV 26505 U.S.A.
Rx only
S-11367 R1
Packaged and Distributed by:
Mylan Institutional Inc.
Rockford, IL 61103 U.S.A.
This unit dose package is not child resistant.
For institutional use only.
Keep this and all drugs out of the reach of children.
This container provides light-resistance.
See window for lot number and expiration date.