NDC Code(s) : 51531-2326-1, 51531-2326-5
Packager : Mary Kay Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Mary Kay TimeWise Day Solution Sunscreen SPF 25octinoxate, octisalate, zinc oxide, oxybenzone, LOTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
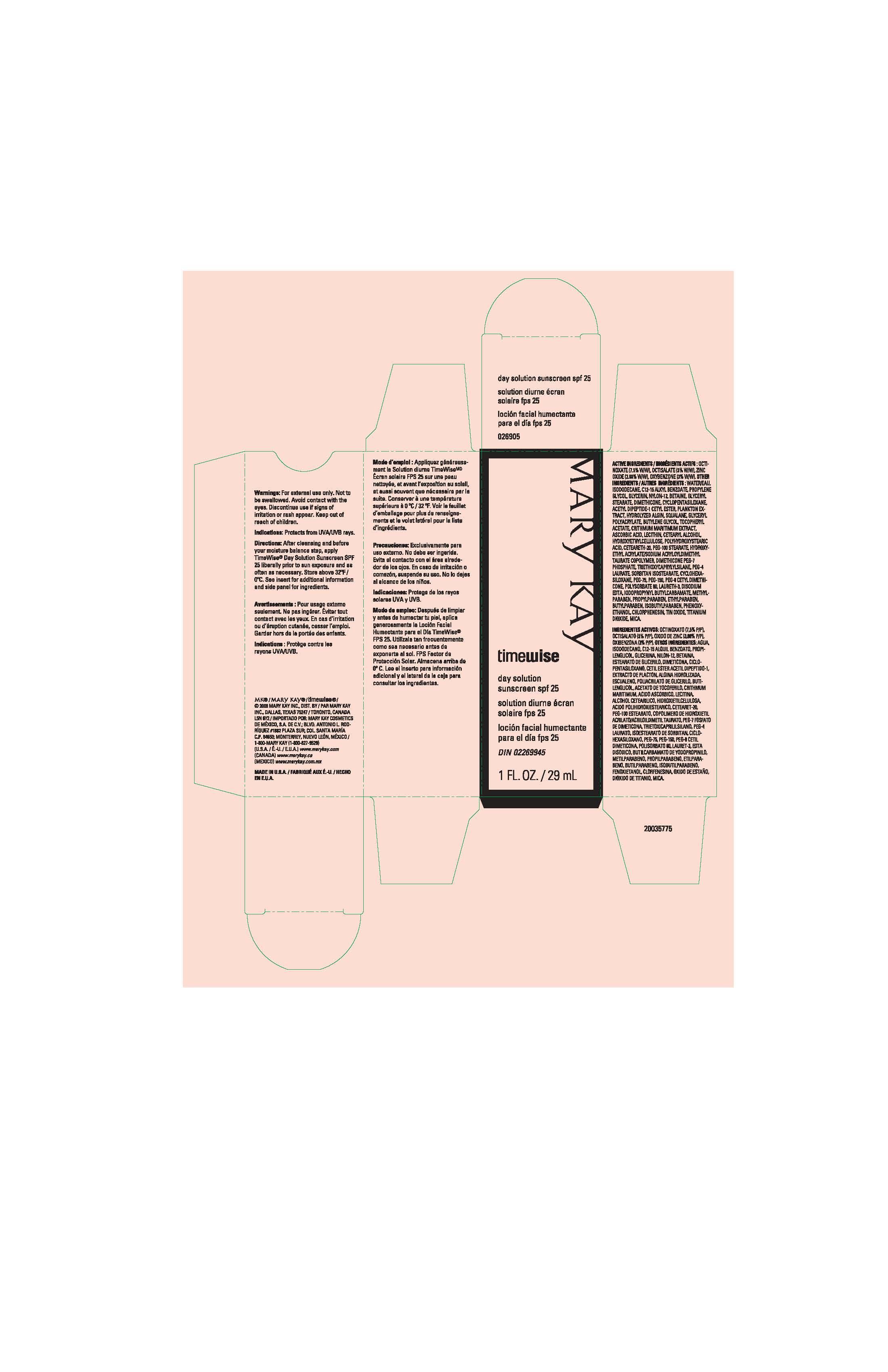
PRINCIPAL DISPLAY PANEL
Mary Kay
timewise
day solution sunscreen spf 25
solution diurne ecran solaire fps 25
locion facial humectante para el dia fps 25
1 FL. OZ. / 29 mL