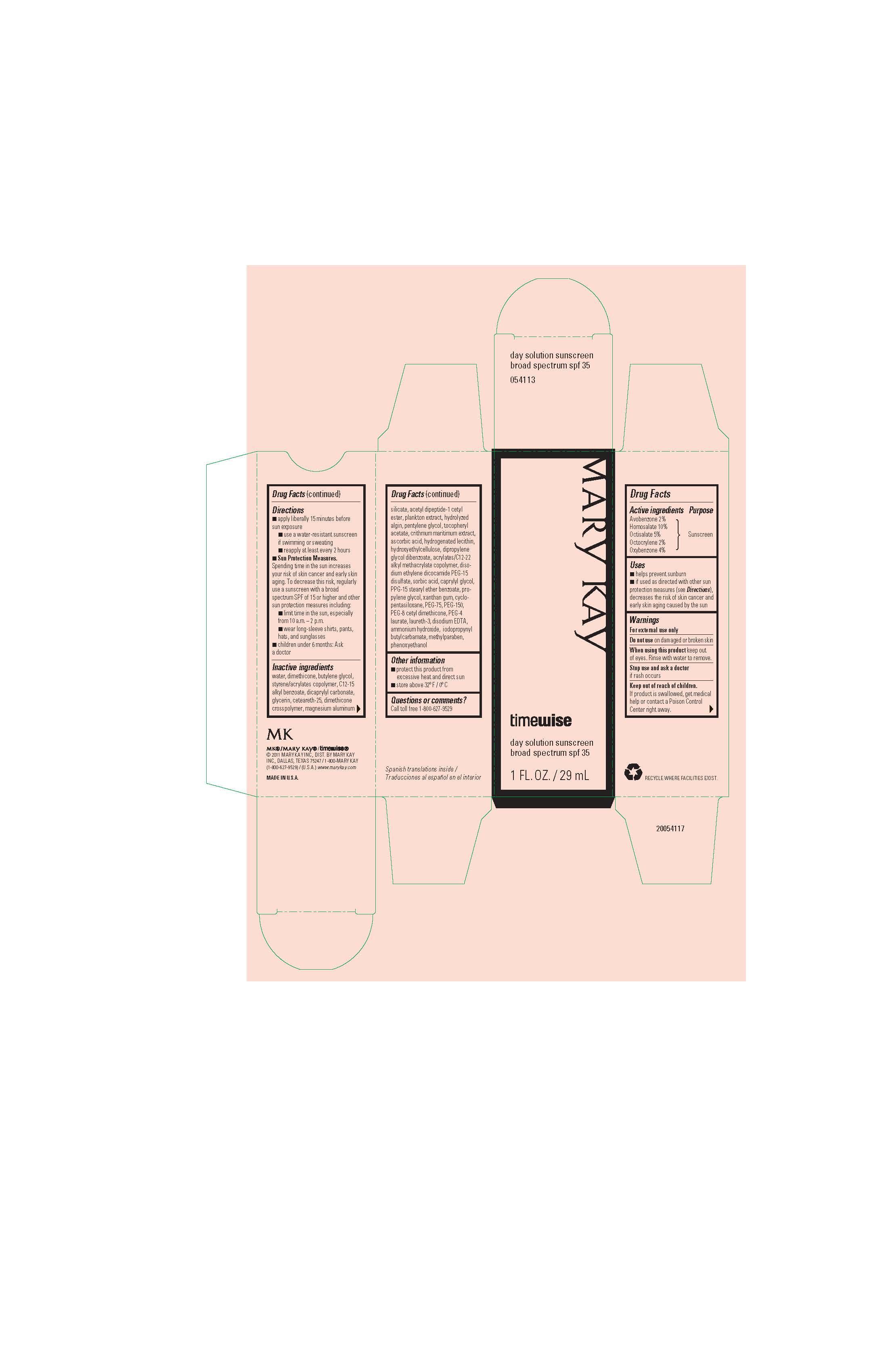
NDC Code(s) : 51531-8360-1, 51531-8360-5
Packager : Mary Kay Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Mary Kay TimeWise Day Solution SPF 35avobenzone, homosalate, octisalate, octocrylene, oxybenzone SOLUTION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Mary Kay
timewise day solution
sunscreen
broad spectrum
spf 35
1 FL. OZ. / 29 mL