NDC Code(s) : 51754-5060-1
Packager : Exela Pharma Sciences, LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Betamethasone Sodium Phosphate and Betamethasone AcetateBetamethasone Sodium Phosphate and Betamethasone Acetate INJECTION, SUSPENSION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Exela Pharma Sciences, LLC(831274399) |
| REGISTRANT - Organon LLC(117494753) |
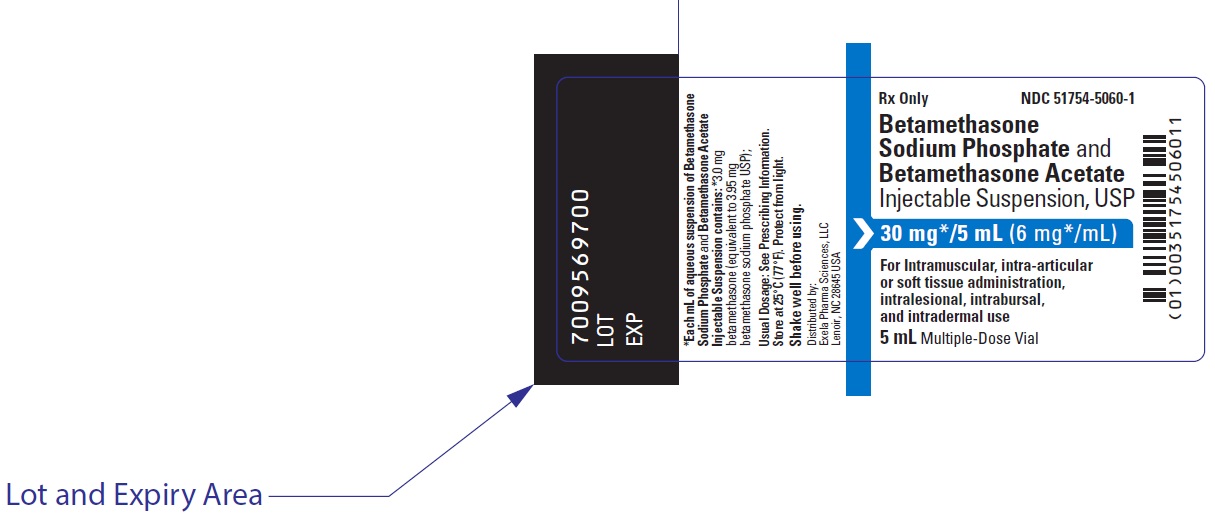
PRINCIPAL DISPLAY PANEL
NDC 51754-5060-1
Rx Only
Betamethasone
Sodium Phosphate and
Betamethasone Acetate
Injectable Suspension, USP
30 mg */5 mL (6 mg*/mL)
For intramuscular,
intra-articular or soft tissue
administration, intralesional,
intrabursal, and intradermal use
Shake well before using.
5 mL Multiple-Dose Vial
EXELA
PHARMA SCIENCES
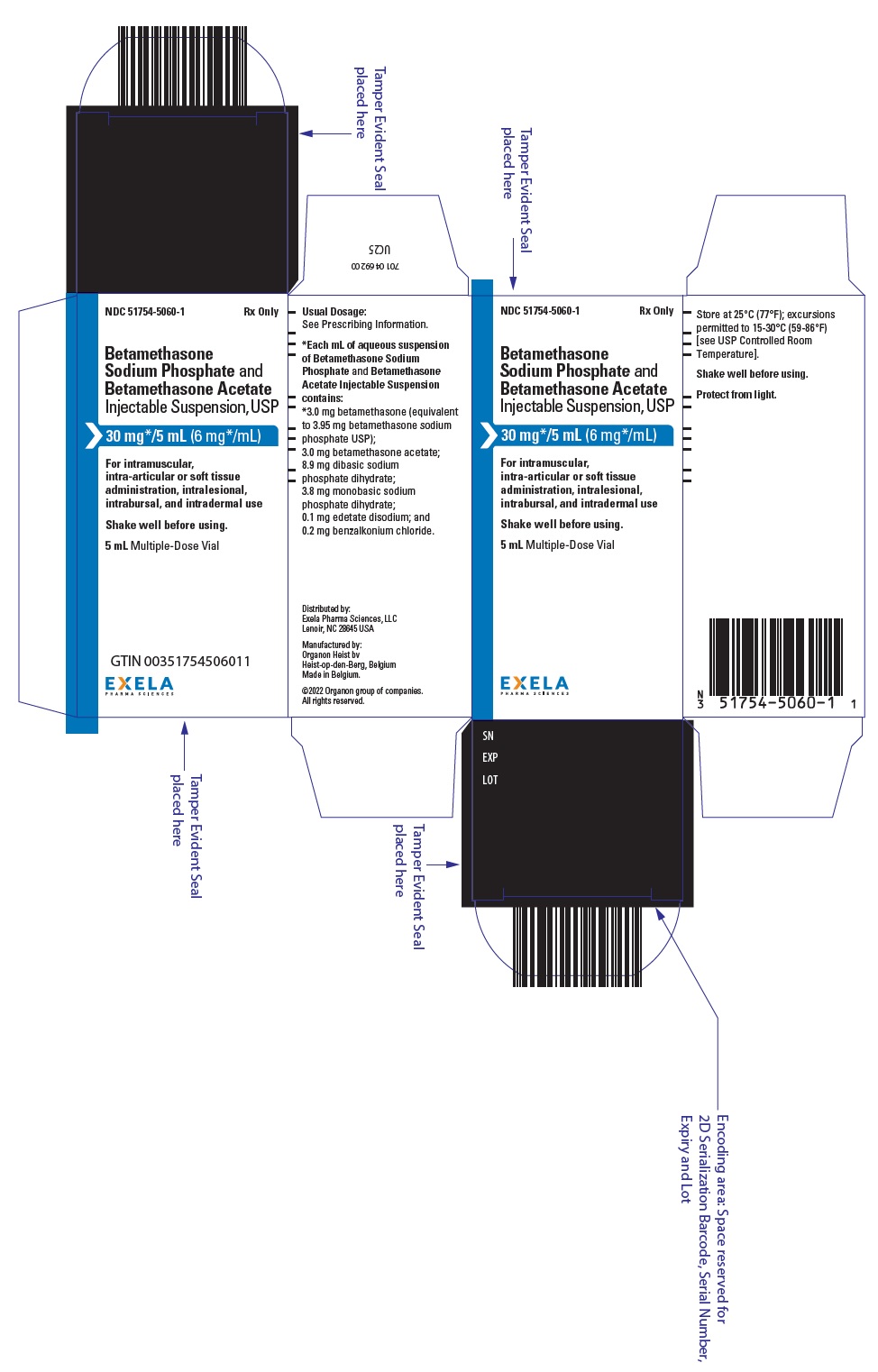
PRINCIPAL DISPLAY PANEL
Rx Only
NDC 51754-5060-1
Betamethasone
Sodium Phosphate and
Betamethasone Acetate
Injectable Suspension, USP
30 mg */5 mL (6 mg */mL)
For Intramuscular, intra-articular
or soft tissue administration,
intralesional, intrabursal,
and intradermal use
5 mL Multiple-Dose Vial