NDC Code(s) : 51817-170-01
Packager : Pharmascience Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Busulfanbusulfan INJECTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Pharmascience Inc.(202657094) |
| REGISTRANT - Pharmascience Inc.(244596946) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Pharmascience Inc. | 202657094 | manufacture(51817-170) | |
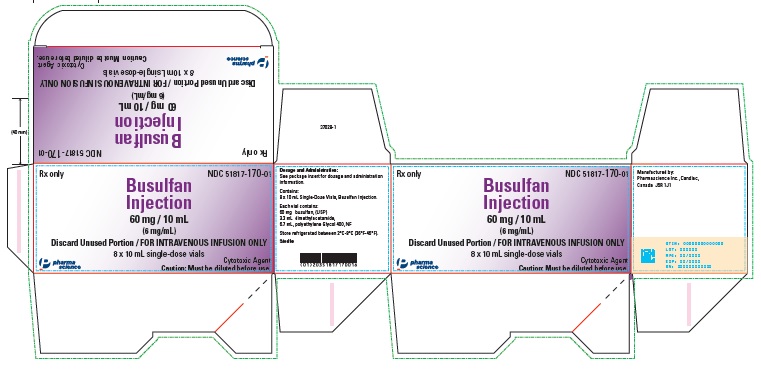
PRINCIPAL DISPLAY PANEL
Rx only
NDC 51817-170-01
Sterile
Busulfan
Injection
10 mL (6 mg/mL)
Single-Dose Vials
Cytotoxic Agent
Caution: Must be diluted before use.