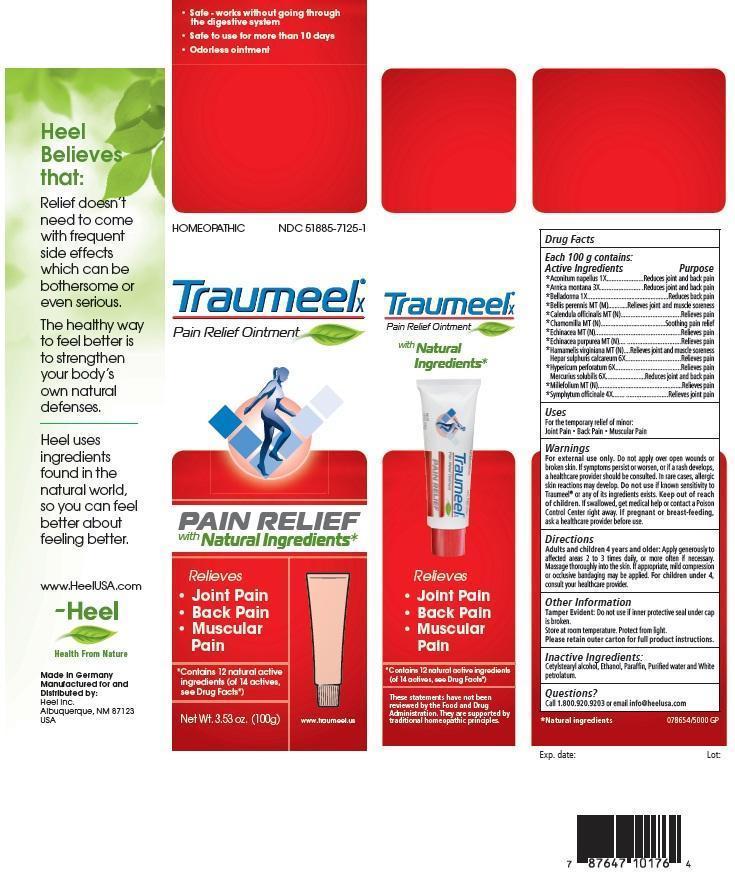
NDC Code(s) : 51885-7125-3, 51885-7125-1, 51885-7125-6
Packager : Biologische Heilmittel Heel
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Traumeel X CALENDULA OFFICINALIS FLOWERING TOP and HAMAMELIS VIRGINIANA ROOT BARK/STEM BARK and ARNICA MONTANA ROOT and ACONITUM NAPELLUS and ATROPA BELLADONNA and BELLIS PERENNIS and MATRICARIA RECUTITA and ECHINACEA, UNSPECIFIED and ECHINACEA PURPUREA and ACHILLEA MILLEFOLIUM and CALCIUM SULFIDE and MERCURIUS SOLUBILIS and COMFREY ROOT and HYPERICUM PERFORATUM and OINTMENT | ||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL