NDC Code(s) : 52125-624-60
Packager : REMEDYREPACK INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Permethrin Permethrin CREAM | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
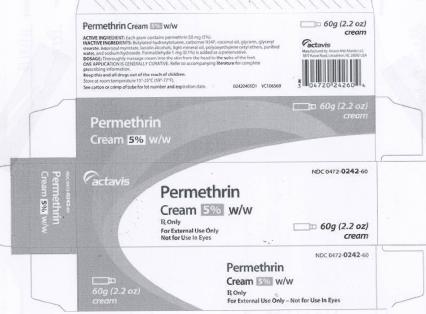
PRINCIPAL DISPLAY PANEL
DRUG: Permethrin
GENERIC: Permethrin
DOSAGE: CREAM
ADMINSTRATION: TOPICAL
NDC: 52125-624-60
ACTIVE INGREDIENT(S):
- PERMETHRIN 50mg in 1g
INACTIVE INGREDIENT(S):
- BUTYLATED HYDROXYTOLUENE
- CETETH-10
- WATER
- CETETH-20
- LIGHT MINERAL OIL
- SODIUM HYDROXIDE
- FORMALDEHYDE
- CARBOMER HOMOPOLYMER (ALLYL SUCROSE CROSSLINKED)
- COCONUT OIL
- GLYCERIN
- GLYCERYL MONOSTEARATE
- ISOPROPYL MYRISTATE
- LANOLIN ALCOHOLS
PACKAGING: 60 g in 1 TUBE