NDC Code(s) : 52125-636-01
Packager : REMEDYREPACK INC.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Diphenhydramine Hydrochloridediphenhydramine hydrochloride INJECTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
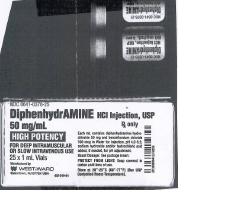
PRINCIPAL DISPLAY PANEL
DRUG: Diphenhydramine Hydrochloride
GENERIC: diphenhydramine hydrochloride
DOSAGE: INJECTION
ADMINSTRATION: INTRAMUSCULAR
NDC: 52125-636-01
ACTIVE INGREDIENT(S):
- DIPHENHYDRAMINE HYDROCHLORIDE 50mg in 1mL
INACTIVE INGREDIENT(S):
- BENZETHONIUM CHLORIDE
- SODIUM HYDROXIDE
- HYDROCHLORIC ACID
- WATER
PACKAGING: 1 mL in 1 VIAL