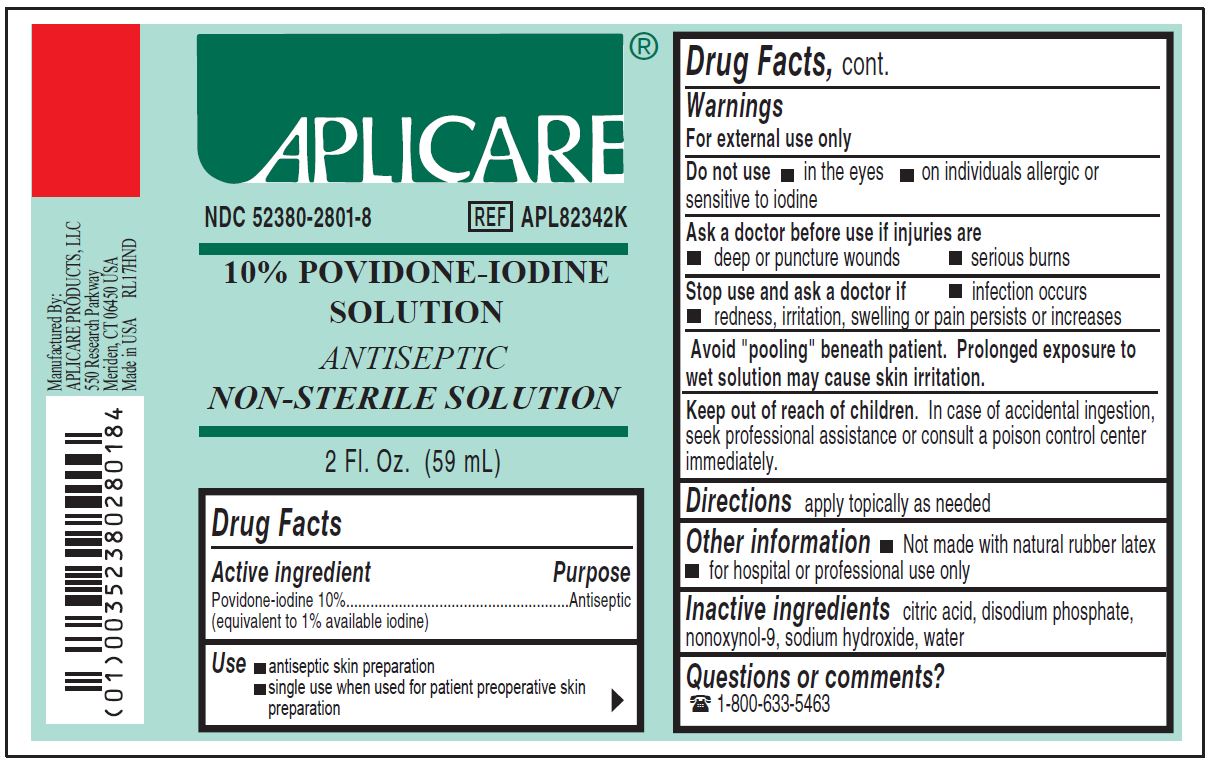
NDC Code(s) : 52380-2801-8
Packager : Aplicare Products, LLC
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Aplicare Povidone-IodinePovidone-Iodine SOLUTION | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| LABELER - Aplicare Products, LLC(081054904) |
| REGISTRANT - Medline Industries, LP(025460908) |
PRINCIPAL DISPLAY PANEL