NDC Code(s) : 52544-276-21, 52544-290-21, 52544-249-28, 52544-233-28
Packager : Actavis Pharma, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| MicrogestinNorethindrone Acetate and Ethinyl Estradiol TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MicrogestinNorethindrone Acetate and Ethinyl Estradiol TABLET | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| MicrogestinNorethindrone Acetate and Ethinyl Estradiol and Ferrous Fumarate KIT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MicrogestinNorethindrone Acetate and Ethinyl Estradiol and Ferrous Fumarate KIT | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
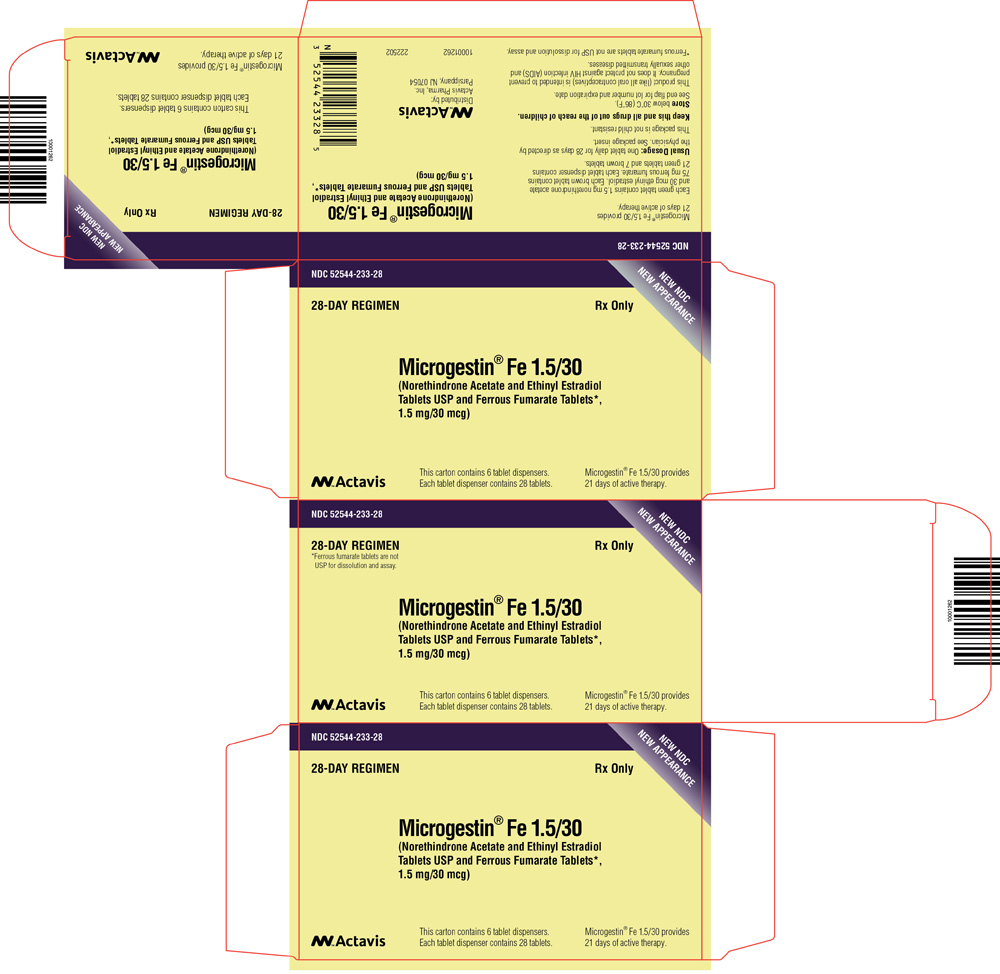
PRINCIPAL DISPLAY PANEL
NDC 52544-276-21
MICROGESTIN
®1/20
(Norethindrone Acetate and Ethinyl Estradiol
Tablets USP)
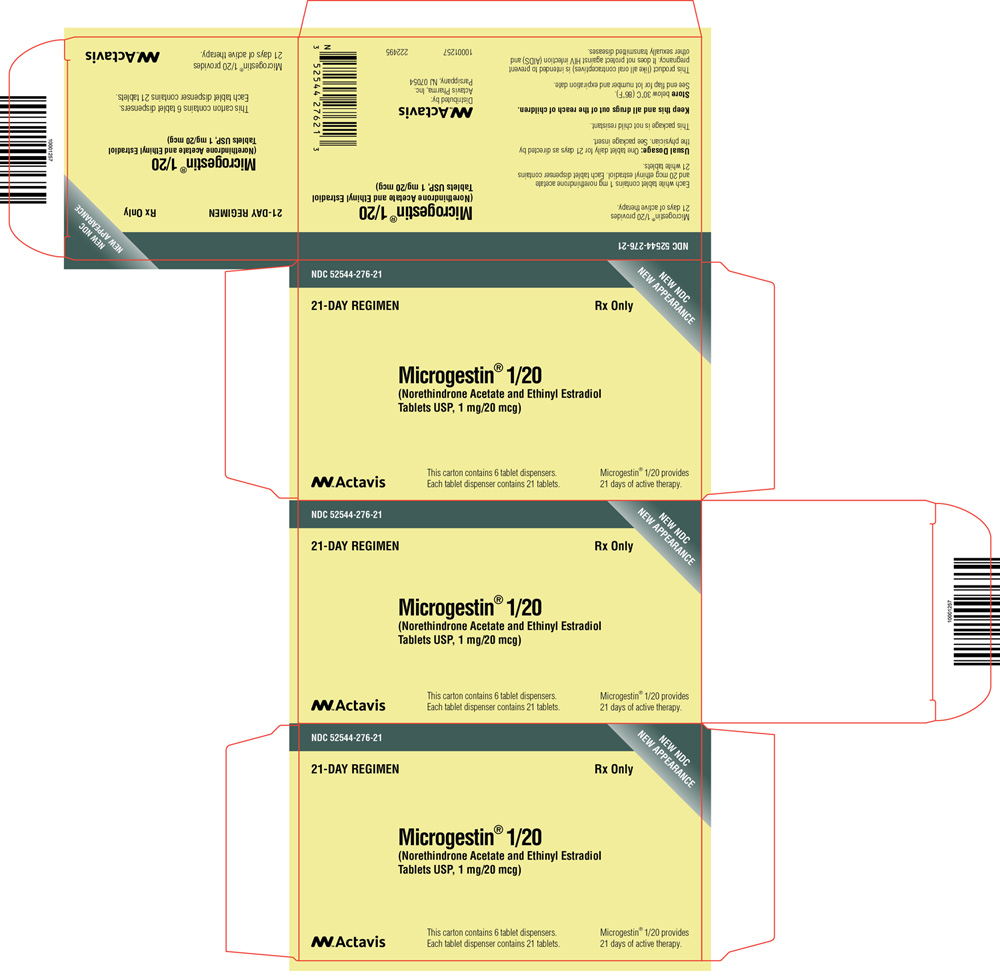
PRINCIPAL DISPLAY PANEL
NDC 52544-249-28
MICROGESTIN® Fe 1/20
(Norethindrone Acetate and Ethinyl Estradiol
Tablets USP and Ferrous Fumarate Tablets*)
*ferrous fumarate tablets are not USP for dissolution and assay
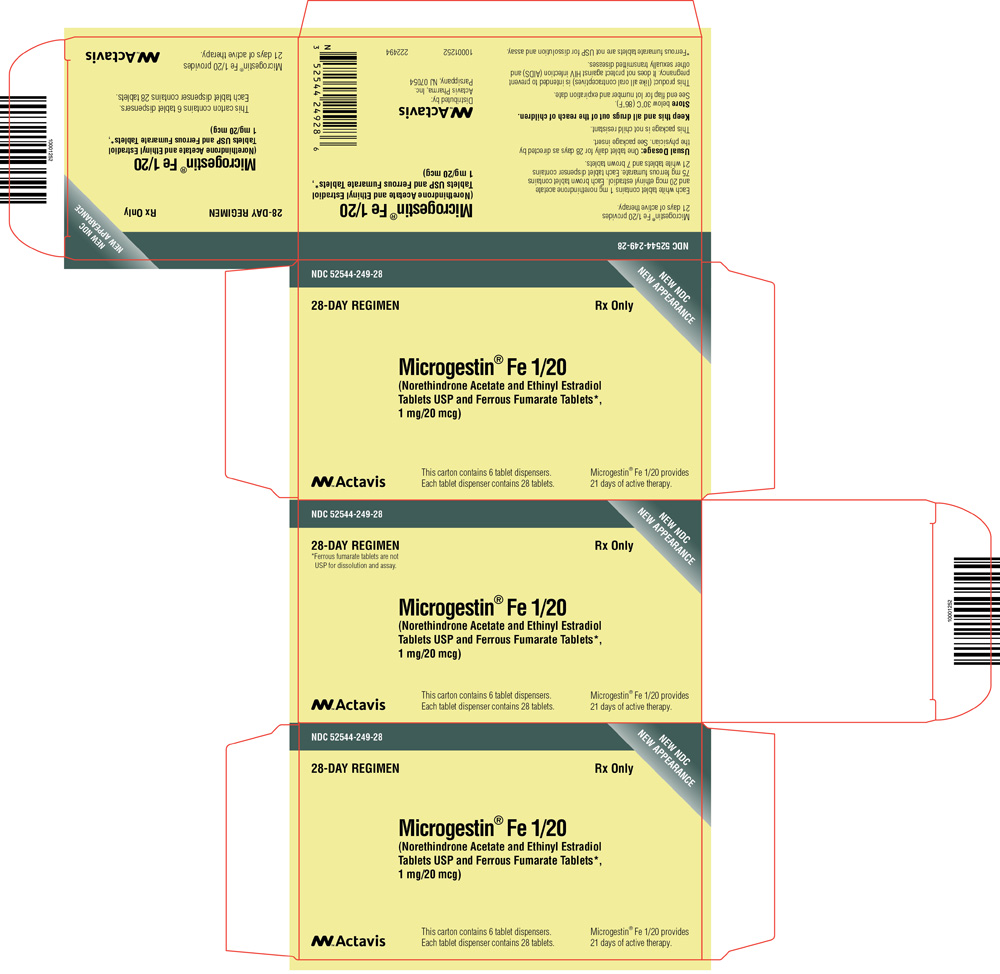
PRINCIPAL DISPLAY PANEL
NDC 52544-290-21
MICROGESTIN® 1.5/30
(Norethindrone Acetate and Ethinyl Estradiol
Tablets USP)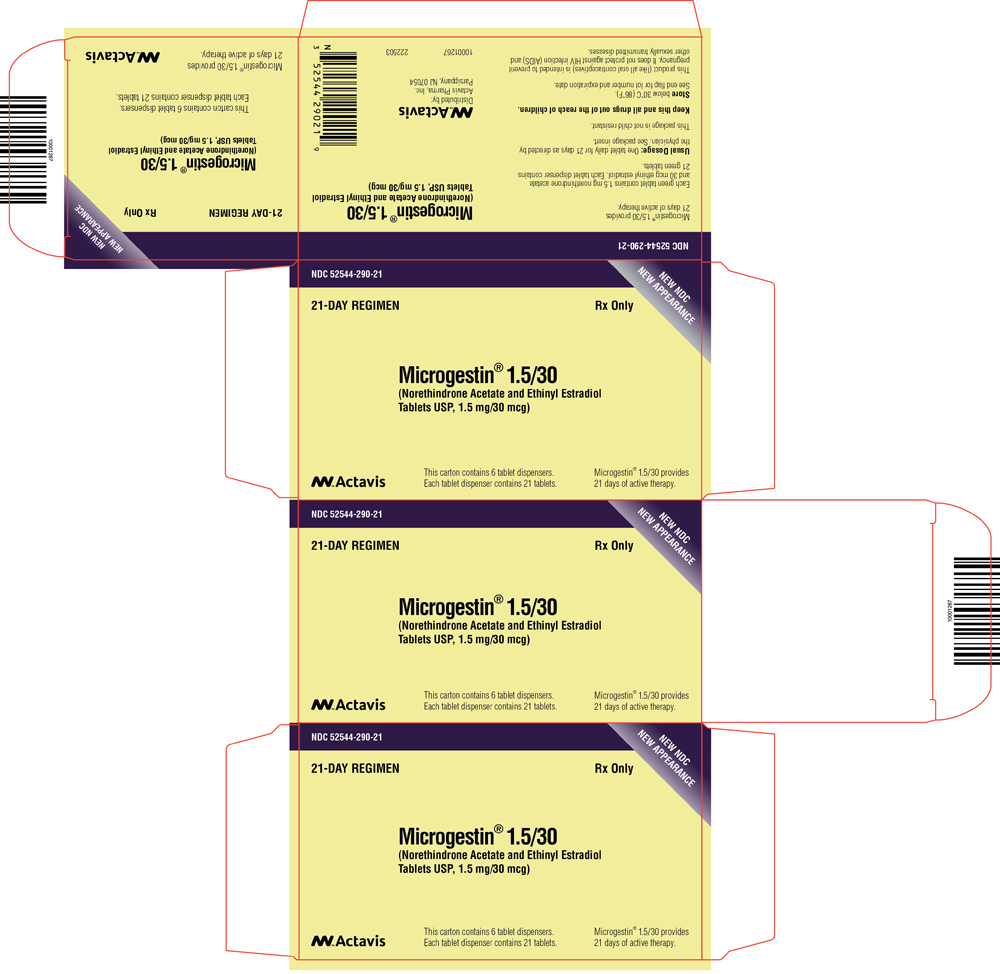
PRINCIPAL DISPLAY PANEL
NDC 52544-233-28
MICROGESTIN® Fe 1.5/30
(Norethindrone Acetate and Ethinyl Estradiol
Tablets USP and Ferrous Fumarate Tablets*)
*ferrous fumarate tablets are not USP for dissolution and assay