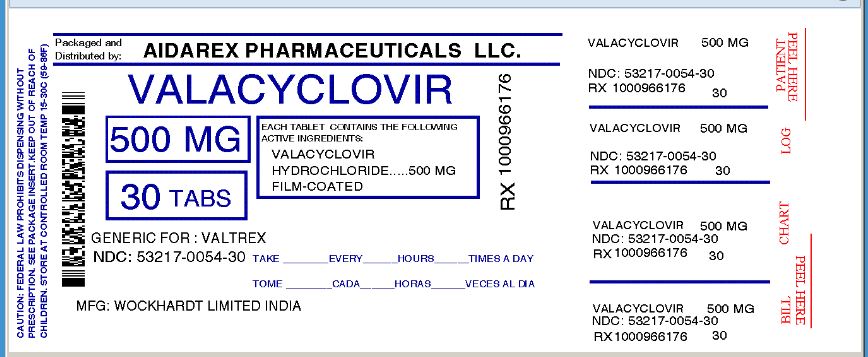
NDC Code(s) : 53217-054-30, 53217-054-60, 53217-054-90
Packager : Aidarex Pharmaceuticals LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| VALACYCLOVIR HYDROCHLORIDEVALACYCLOVIR HYDROCHLORIDE TABLET, FILM COATED | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Aidarex Pharmaceuticals LLC(801503249) |
PRINCIPAL DISPLAY PANEL