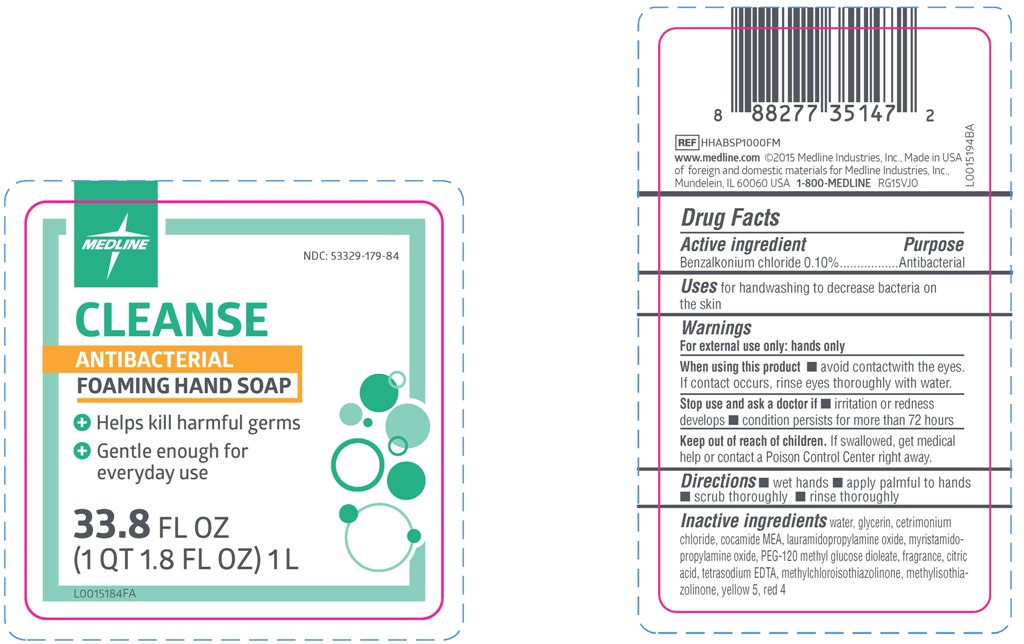
NDC Code(s) : 53329-179-84
Packager : Medline Industries, Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CleanseBenzalkonium Chloride 0.10% SOAP | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
CLEANSE ANTIBACTERIAL HAND SOAP
- Helps kill harmful germs
- Gentle enough for everyday use