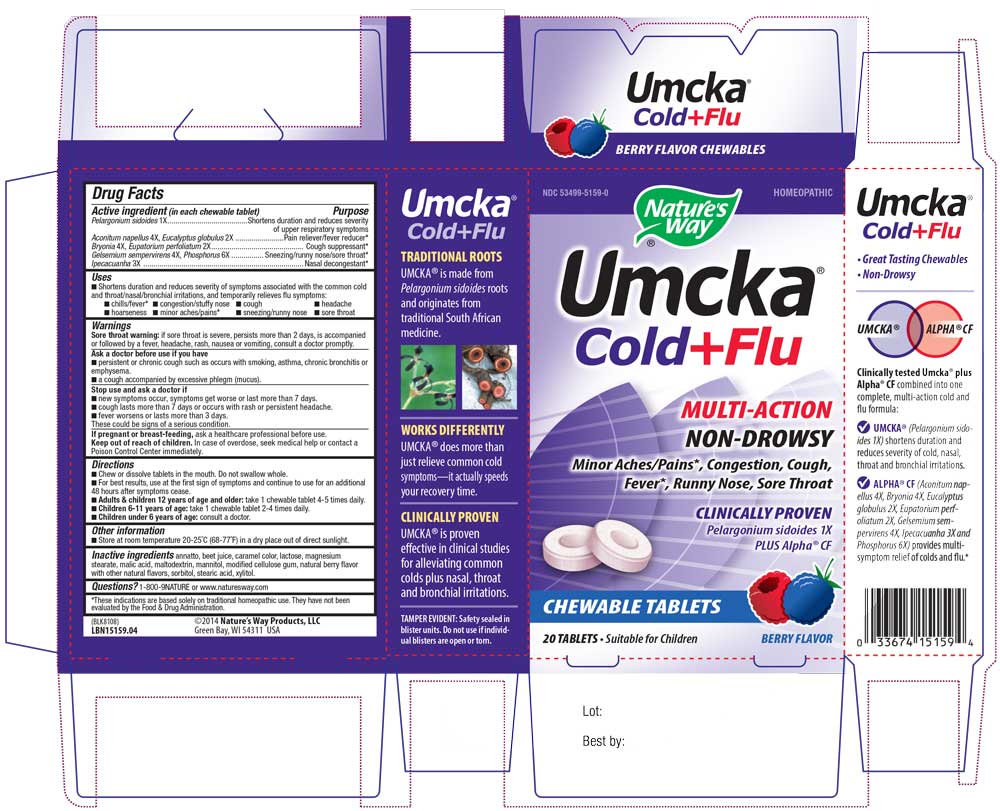
NDC Code(s) : 53499-5159-0
Packager : Schwabe North America
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Umcka Cold Flu BerryPelargonium sidoides, Aconitum Napellus, Bryonia, Eucalyptus Globulus, Eupatorium Perfoliatum, Gelsemium Sempervirens, Ipecacuanha, Phosphorus TABLET, CHEWABLE | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
15159_04 UMCKA CF Berry Chew.jpg