NDC Code(s) : 53675-132-01
Packager : Aruba Aloe Balm NV
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Aruba Aloe Deodorant Men Aluminum Chlorohydrate LOTION | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
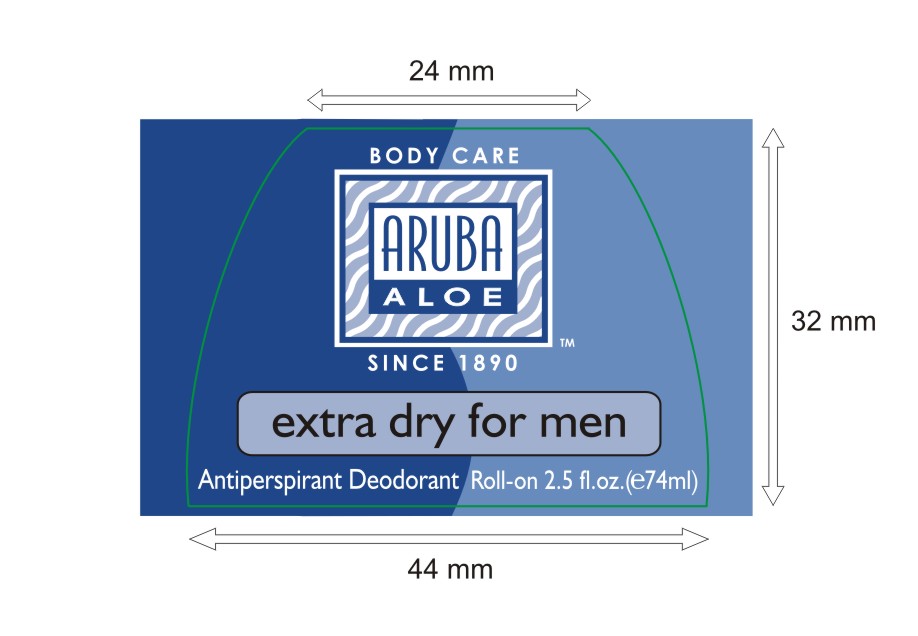
PRINCIPAL DISPLAY PANEL
extra dry men
Anrtiperspirant Deodorant Roll-on 2 fl. oz (e 59ml)