NDC Code(s) : 54505-101-01, 54505-101-02, 54505-102-01, 54505-102-02
Packager : Lineage Therapeutics Inc
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| epinephrineepinephrine INJECTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| epinephrineepinephrine INJECTION | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
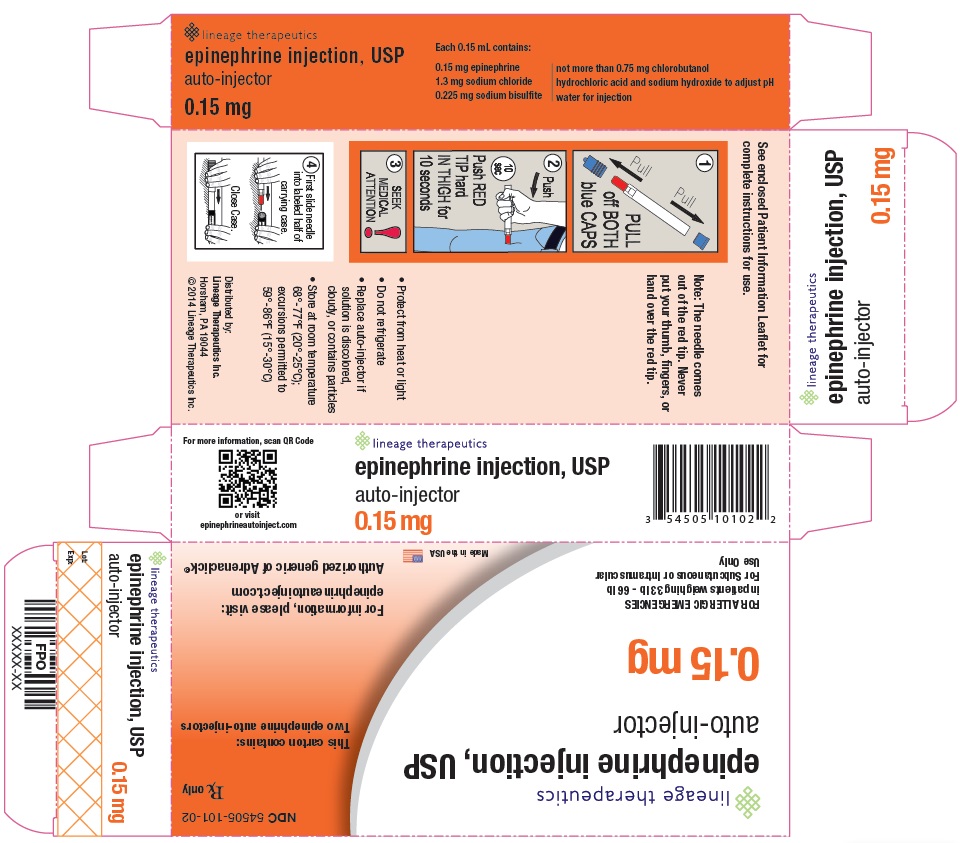
PRINCIPAL DISPLAY PANEL - 0.15 mg CARTON NDC 54505-101-02
Rx Only
Lineage Therapeutics
epinephrine injection, USP
auto-injector
0.15 mg
For Allergic Emergencies
in patients weighing 33 lb – 66 lb
For Subcutaneous or Intramuscular Use Only
This carton contains: Two epinephrine auto-injectors
PRINCIPAL DISPLAY PANEL
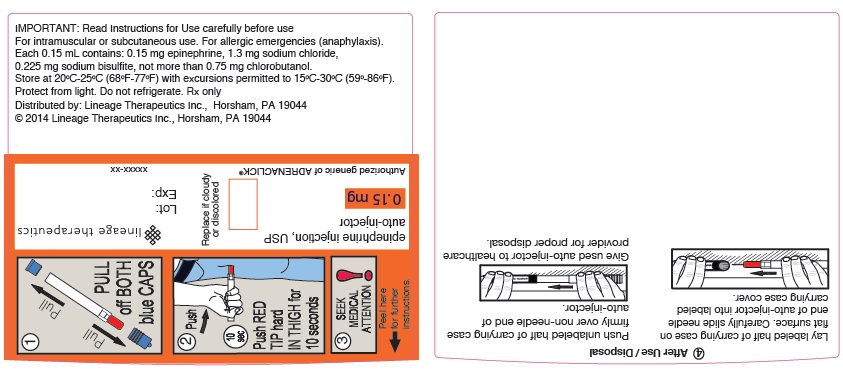
PRINCIPAL DISPLAY PANEL - 0.15 mg WRAP LABEL
Lineage Therapeutics
epinephrine injection, USP
auto-injector
0.15 mg
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 0.15 mg CASE LABEL
epinephrine injection, USP
auto-injector
0.15 mg
Lineage Therapeutics

PRINCIPAL DISPLAY PANEL
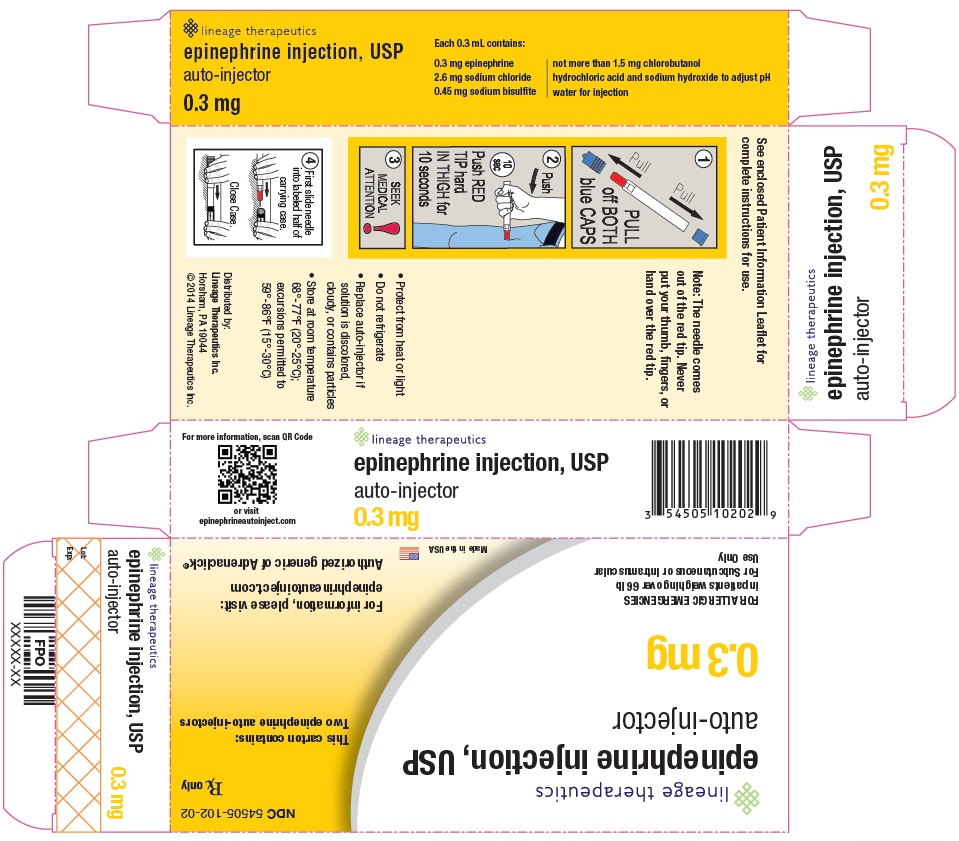
PRINCIPAL DISPLAY PANEL - 0.3 mg CARTON NDC 54505-102-02
Rx Only
Lineage Therapeutics
epinephrine injection, USP
auto-injector
0.3 mg
For Allergic Emergencies
in patients weighing over 66 lb
For Subcutaneous or Intramuscular Use Only
This carton contains: Two epinephrine auto-injectors
PRINCIPAL DISPLAY PANEL
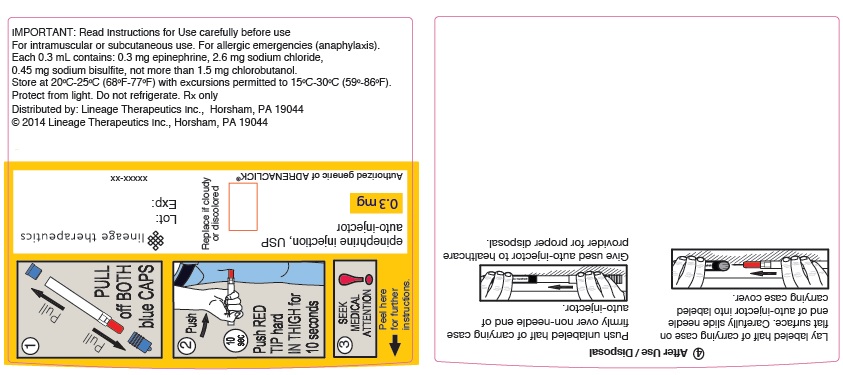
PRINCIPAL DISPLAY PANEL - 0.3 mg WRAP LABEL
Lineage Therapeutics
epinephrine injection, USP
auto-injector
0.3 mg
PRINCIPAL DISPLAY PANEL
PRINCIPAL DISPLAY PANEL - 0.3 mg CASE LABEL
epinephrine injection, USP
auto-injector
0.3 mg
Lineage Therapeutics










