NDC Code(s) : 54565-2202-2, 54565-2202-7, 54565-2202-1
Packager : Les Emballages Knowlton Inc.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ban Aluminum Chlorohydrate LIQUID | ||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
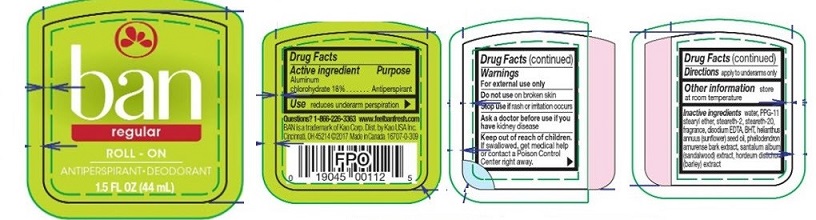
PRINCIPAL DISPLAY PANEL
ban
regular
ROLL-ON
ANTIPERSPIRANT·DEODORANT
1.5 FL OZ (44 mL)