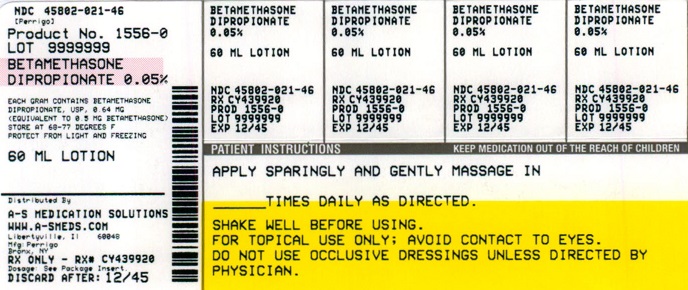
NDC Code(s) : 54569-1556-0
Packager : A-S Medication Solutions LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Betamethasone DipropionateBetamethasone Dipropionate LOTION | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 54569-1556-0
Relabeled by:
A-S Medication Solutions
Libertyville, IL 60048