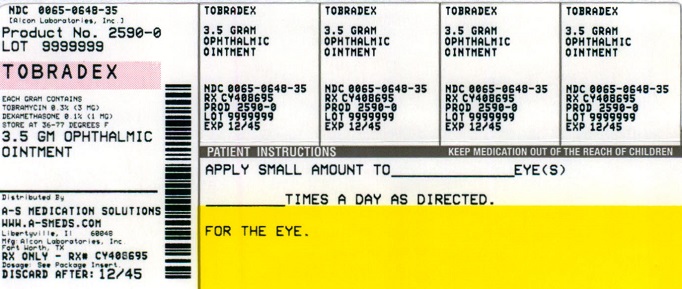
NDC Code(s) : 54569-2590-0
Packager : A-S Medication Solutions LLC
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TobraDextobramycin and dexamethasone OINTMENT | |||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
PRINCIPAL DISPLAY PANEL
NDC 54569-2590-0
Relabeled by:
A-S Medication Solutions
Libertyville, IL 60048