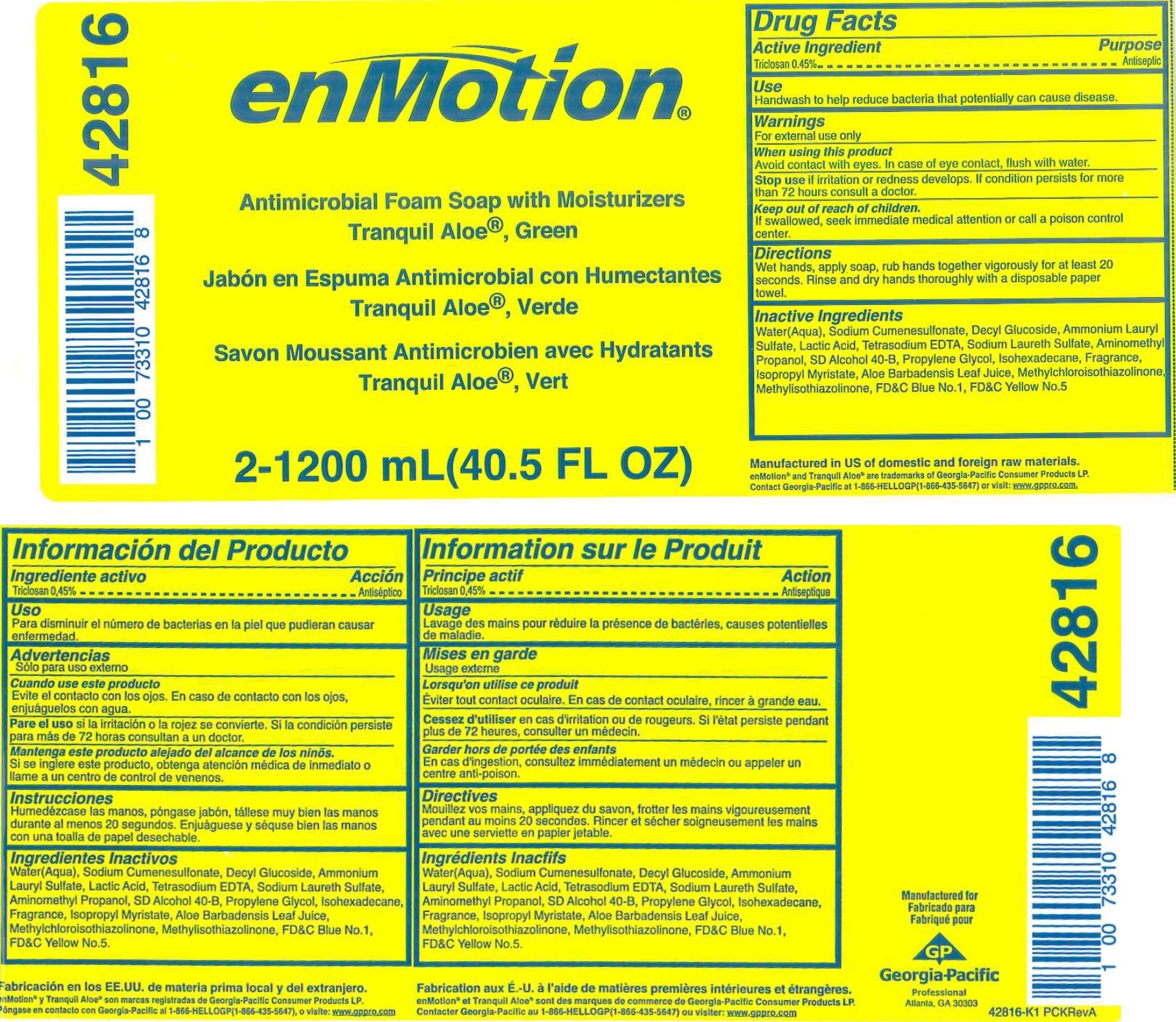
NDC Code(s) : 54622-054-00
Packager : Georgia-Pacific Consumer Products LP
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| enMotion Antimicrobial FoamTRICLOSAN SOAP | ||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL