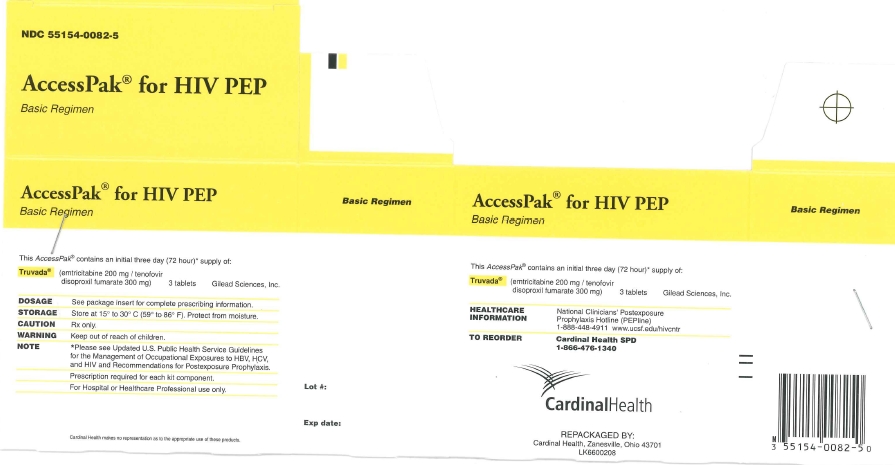
NDC Code(s) : 55154-0082-5
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| TRUVADAemtricitabine and tenofovir disoproxil fumarate TABLET | ||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Truvada®
3 Tablets
(emtricitabine and tenofovir disoproxil fumarate) tablets
200 mg/ 300 mg

PRINCIPAL DISPLAY PANEL
NDC 55154-0082-5
AccessPak® for HIV PEP
Basic Regimen