NDC Code(s) : 55154-3342-0, 55154-3343-0, 55154-3384-0, 55154-3344-0
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Ziprasidone HydrochlorideZiprasidone Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| Ziprasidone HydrochlorideZiprasidone Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
| Ziprasidone HydrochlorideZiprasidone Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Ziprasidone HydrochlorideZiprasidone Hydrochloride CAPSULE | ||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Ziprasidone Hydrochloride
Capsules 20 mg*
10 Capsules

PRINCIPAL DISPLAY PANEL
Ziprasidone Hydrochloride
Capsules 40 mg*
10 Capsules

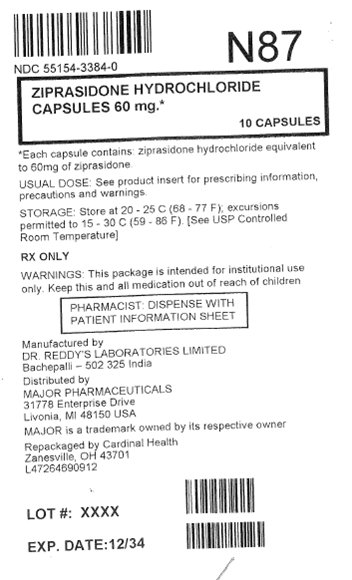
PRINCIPAL DISPLAY PANEL
Ziprasidone Hydrochloride
Capsules 60 mg*
10 Capsules
PRINCIPAL DISPLAY PANEL
Ziprasidone Hydrochloride
Capsules 80 mg*
10 Capsules










