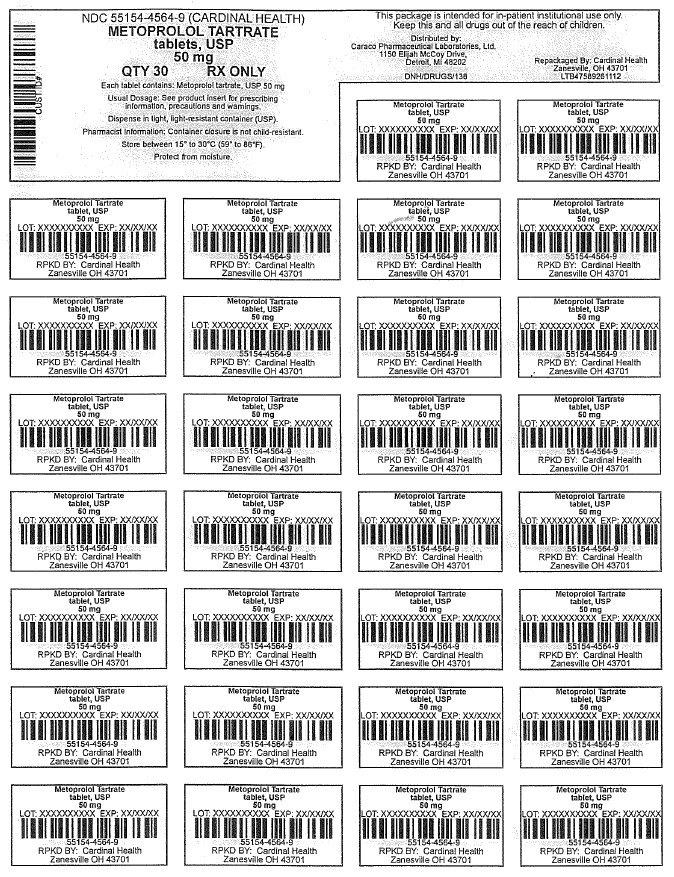
NDC Code(s) : 55154-4564-9
Packager : Cardinal Health
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Metoprolol TartrateMetoprolol tartrate TABLET | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
PRINCIPAL DISPLAY PANEL
Metoprolol Tartrate Tablets, USP
50 mg
30 Tablets