NDC Code(s) : 55648-902-01, 55648-902-02, 55648-902-05, 55648-903-01, 55648-903-02, 55648-903-05, 55648-904-01, 55648-904-02, 55648-904-05, 55648-905-01, 55648-905-04
Packager : Wockhardt Limited
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CAPTOPRILCAPTOPRIL TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Wockhardt Limited(650069115) |
| REGISTRANT - Wockhardt Limited(650069115) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Wockhardt Limited | 916489953 | Analysis(55648-902, 55648-903, 55648-904, 55648-905), Manufacture(55648-902, 55648-903, 55648-904, 55648-905), Label(55648-902, 55648-903, 55648-904, 55648-905), Pack(55648-902, 55648-903, 55648-904, 55648-905) | |
INGREDIENTS AND APPEARANCE
| CAPTOPRILCAPTORPIL TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Wockhardt Limited(650069115) |
| REGISTRANT - Wockhardt Limited(650069115) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Wockhardt Limited | 916489953 | Analysis(55648-902, 55648-903, 55648-904, 55648-905), Manufacture(55648-902, 55648-903, 55648-904, 55648-905), Label(55648-902, 55648-903, 55648-904, 55648-905), Pack(55648-902, 55648-903, 55648-904, 55648-905) | |
INGREDIENTS AND APPEARANCE
| CAPTOPRILCAPTORPIL TABLET | |||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| LABELER - Wockhardt Limited(650069115) |
| REGISTRANT - Wockhardt Limited(650069115) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Wockhardt Limited | 916489953 | Analysis(55648-902, 55648-903, 55648-904, 55648-905), Manufacture(55648-902, 55648-903, 55648-904, 55648-905), Label(55648-902, 55648-903, 55648-904, 55648-905), Pack(55648-902, 55648-903, 55648-904, 55648-905) | |
INGREDIENTS AND APPEARANCE
| CAPTOPRILCAPTORPIL TABLET | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Wockhardt Limited(650069115) |
| REGISTRANT - Wockhardt Limited(650069115) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| Wockhardt Limited | 916489953 | Analysis(55648-902, 55648-903, 55648-904, 55648-905), Manufacture(55648-902, 55648-903, 55648-904, 55648-905), Label(55648-902, 55648-903, 55648-904, 55648-905), Pack(55648-902, 55648-903, 55648-904, 55648-905) | |
PRINCIPAL DISPLAY PANEL
DRUG: Captopril
GENERIC: Captopril
DOSAGE: Tablets
ADMINSTRATION: Oral
NDC: 64679-902-01
STRENGTH: 12.5 mg
COLOR: White
SHAPE: Round
SCORE: Scored
SIZE: 5 mm
IMPRINT: W;902
QTY: 100 Tablets

DRUG: Captopril
GENERIC: Captopril
DOSAGE: Tablets
ADMINSTRATION: Oral
NDC: 64679-903-02
STRENGTH: 25 mg
COLOR: White
SHAPE: Round
SCORE: Scored
SIZE: 7 mm
IMPRINT: W;903
QTY: 1000 Tablets

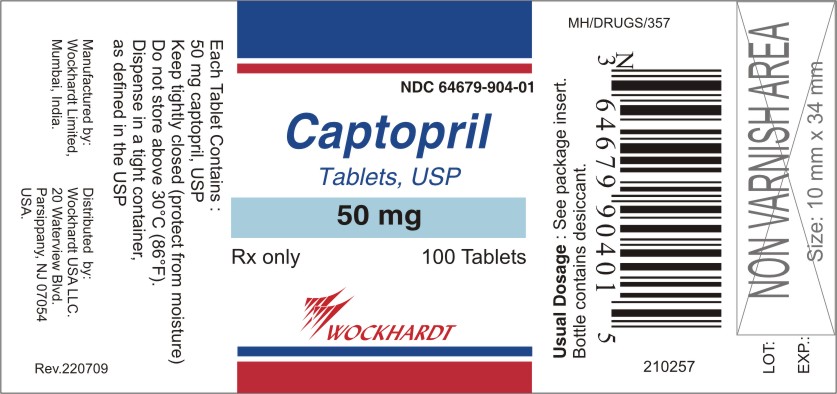
DRUG: Captopril
GENERIC: Captopril
DOSAGE: Tablets
ADMINSTRATION: Oral
NDC: 64679-904-01
STRENGTH: 50 mg
COLOR: White
SHAPE: Round
SCORE: Scored
SIZE: 8 mm
IMPRINT: W;904
QTY: 100 Tablets
DRUG: Captopril
GENERIC: Captopril
DOSAGE: Tablets
ADMINSTRATION: Oral
NDC: 64679-905-02
STRENGTH: 100 mg
COLOR: White
SHAPE: Round
SCORE: Scored
SIZE: 11 mm
IMPRINT: W;905
QTY: 1000 Tablets










