NDC Code(s) : 56104-040-03, 56104-040-30
Packager : Premier Brands of America Inc.
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| Benzethonium chloride Plus Dyclonine hydrochlorideLiquid Bandage LIQUID | ||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| LABELER - Premier Brands of America Inc.(117557458) |
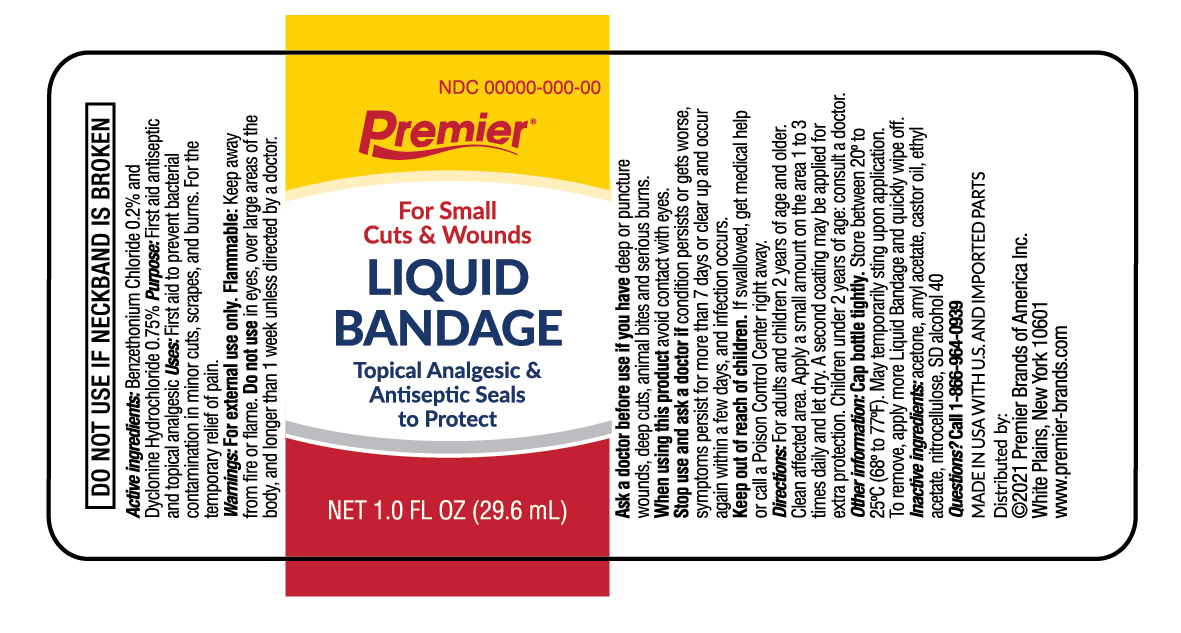
PRINCIPAL DISPLAY PANEL
Premier
For small Cuts & Wounds
Liquid Bandage
Topical Analgesic & Antiseptic
Prevents infection
Invisible & flexible
Easy to use
NET 0.3 FL OZ (9 mL)
USE LIQUID BANDAGE FOR:
Blisters, chapped & cracked finger tips, hangnails, paper cuts, shaving nicks & help in preventing the formation of calluses.
GREAT FOR:
Athletes, active lifestyles, outdoor sportment & musicians.