NDC Code(s) : 57664-239-32, 57664-239-34
Packager : Caraco Pharmaceutical Laboratories, Ltd.
Category : HUMAN PRESCRIPTION DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| NITROFURANTOIN NITROFURANTOIN SUSPENSION | ||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
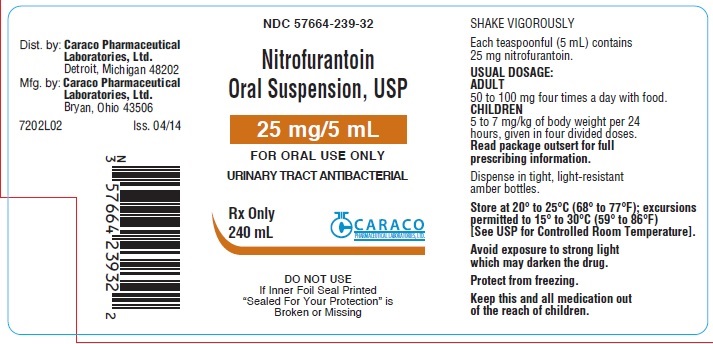
PRINCIPAL DISPLAY PANEL
NDC 57664-239-32
Nitrofurantoin Oral Suspension, USP
25 mg/5 mL
FOR ORAL USE ONLY
URINARY TRACT ANTIBACTERIAL
Rx Only
240 mL
Caraco Pharmaceutical Laboratories, Ltd.