NDC Code(s) : 58411-193-60, 58411-194-60, 58411-195-60, 58411-196-60, 58411-197-60, 58411-198-60, 58411-199-60, 58411-200-60, 58411-201-60, 58411-202-60, 58411-203-60, 58411-204-60
Packager : SHISEIDO AMERICAS CORPORATION
Category : HUMAN OTC DRUG LABEL
DEA Schedule : none
Marketing Status : New Drug Application
INGREDIENTS AND APPEARANCE
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CLE DE PEAU BEAUTE RADIANT FLUID FOUNDATION OCTINOXATE and TITANIUM DIOXIDE CREAM | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LABELER - SHISEIDO AMERICAS CORPORATION(193691821) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
| SHISEIDO AMERICA INC. | 782677132 | manufacture(58411-193, 58411-194, 58411-195, 58411-196, 58411-197, 58411-198, 58411-199, 58411-200, 58411-201, 58411-202, 58411-203, 58411-204), analysis(58411-193, 58411-194, 58411-195, 58411-196, 58411-197, 58411-198, 58411-199, 58411-200, 58411-201, 58411-202, 58411-203, 58411-204) | |
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
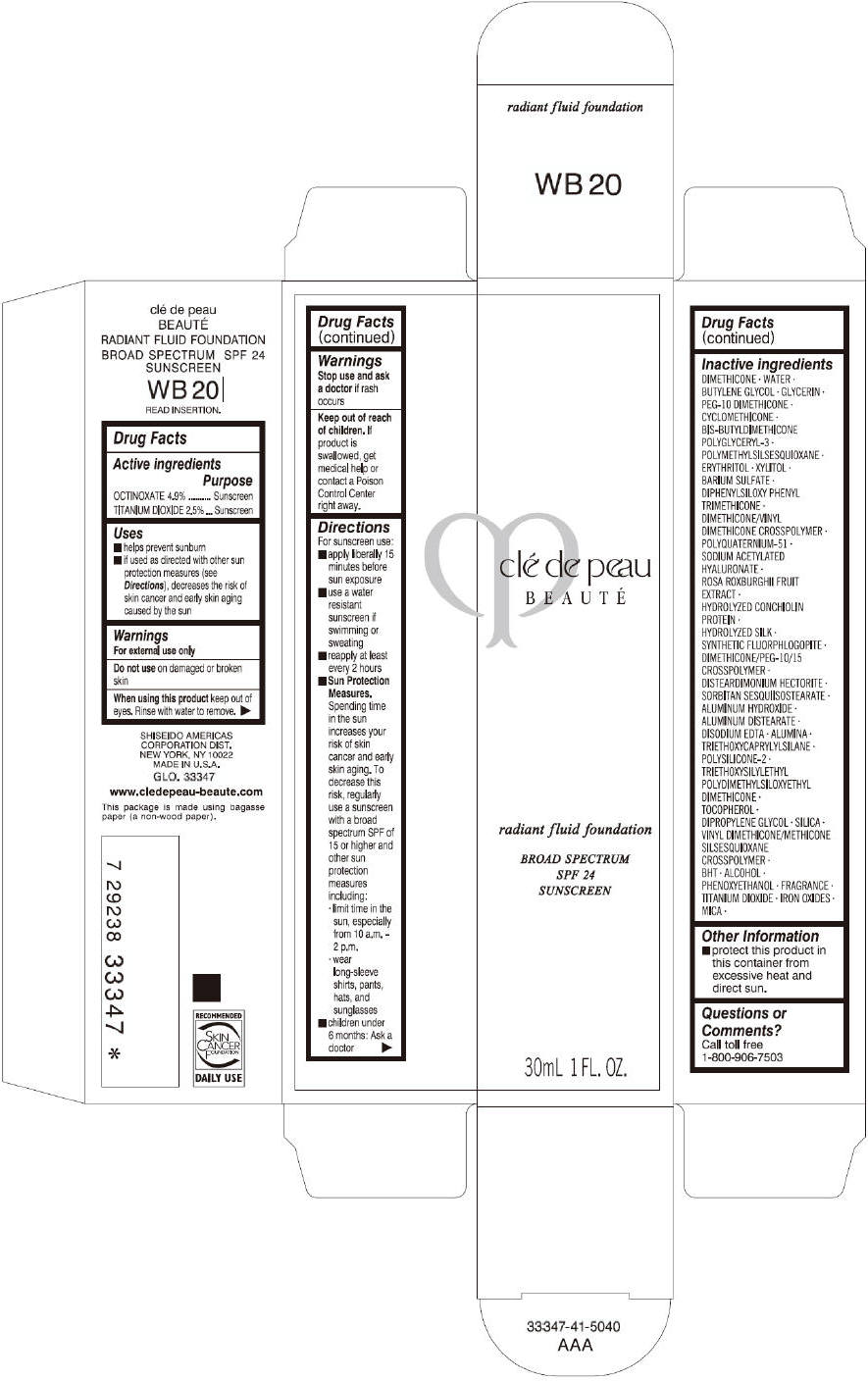
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
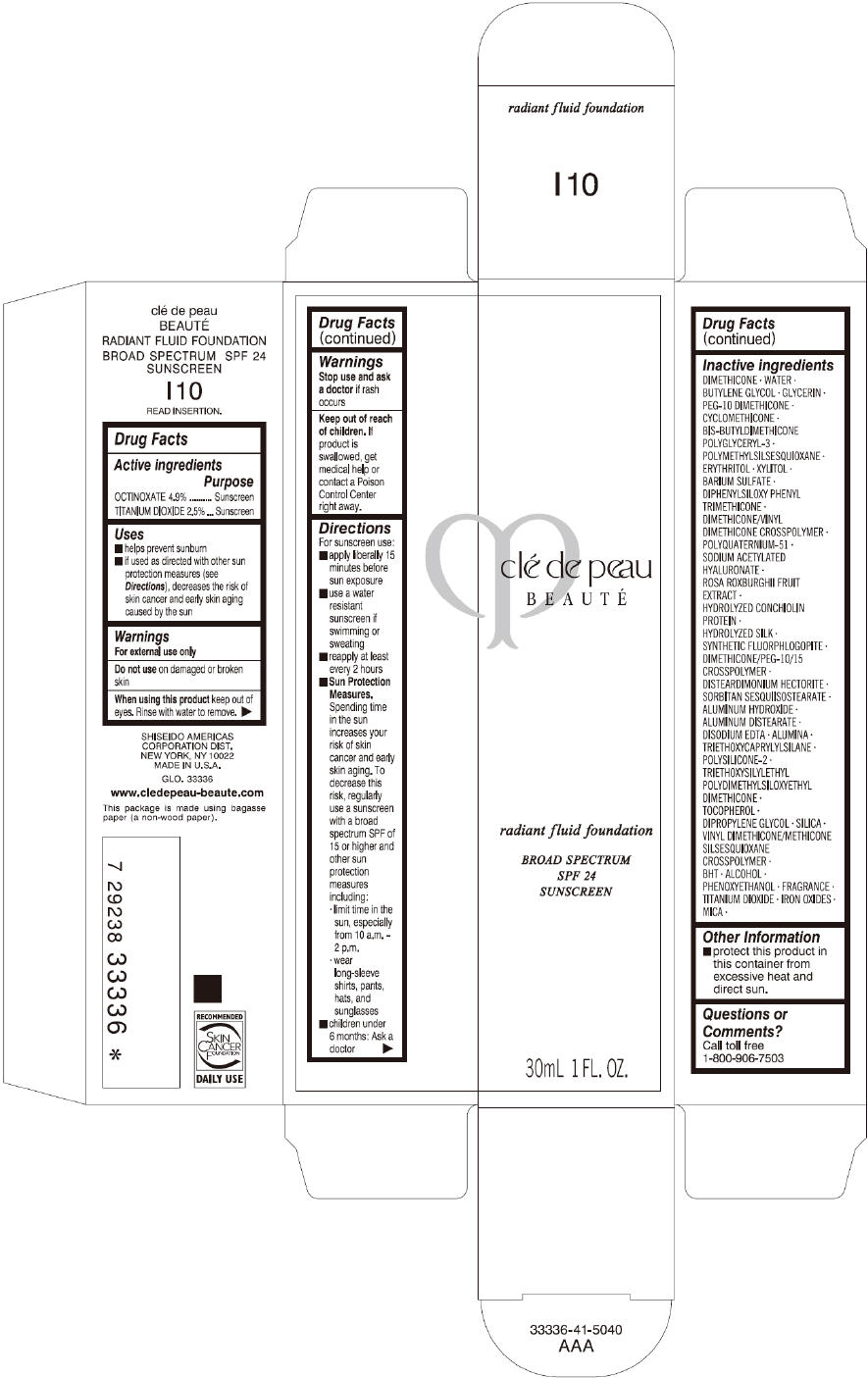
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
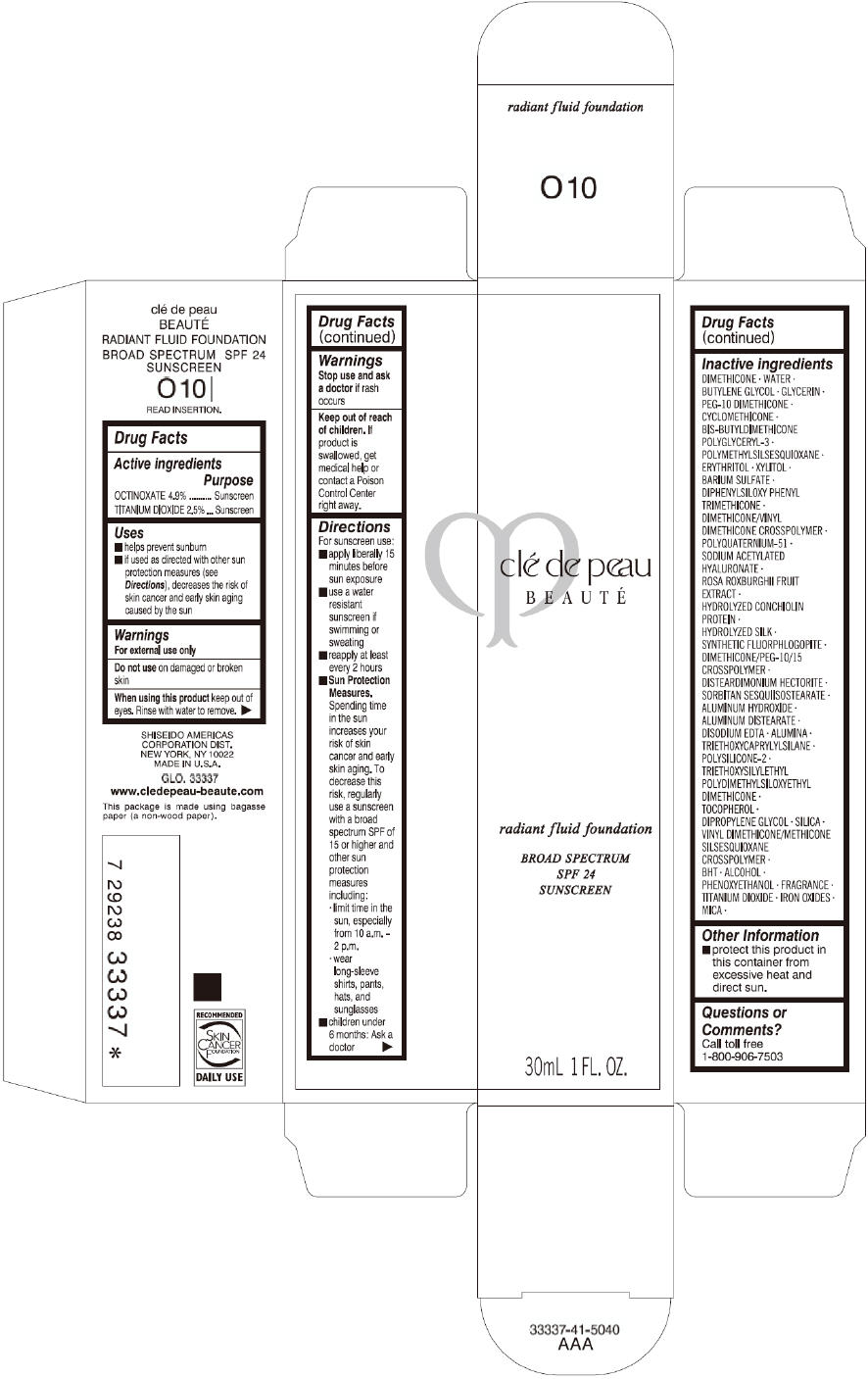
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
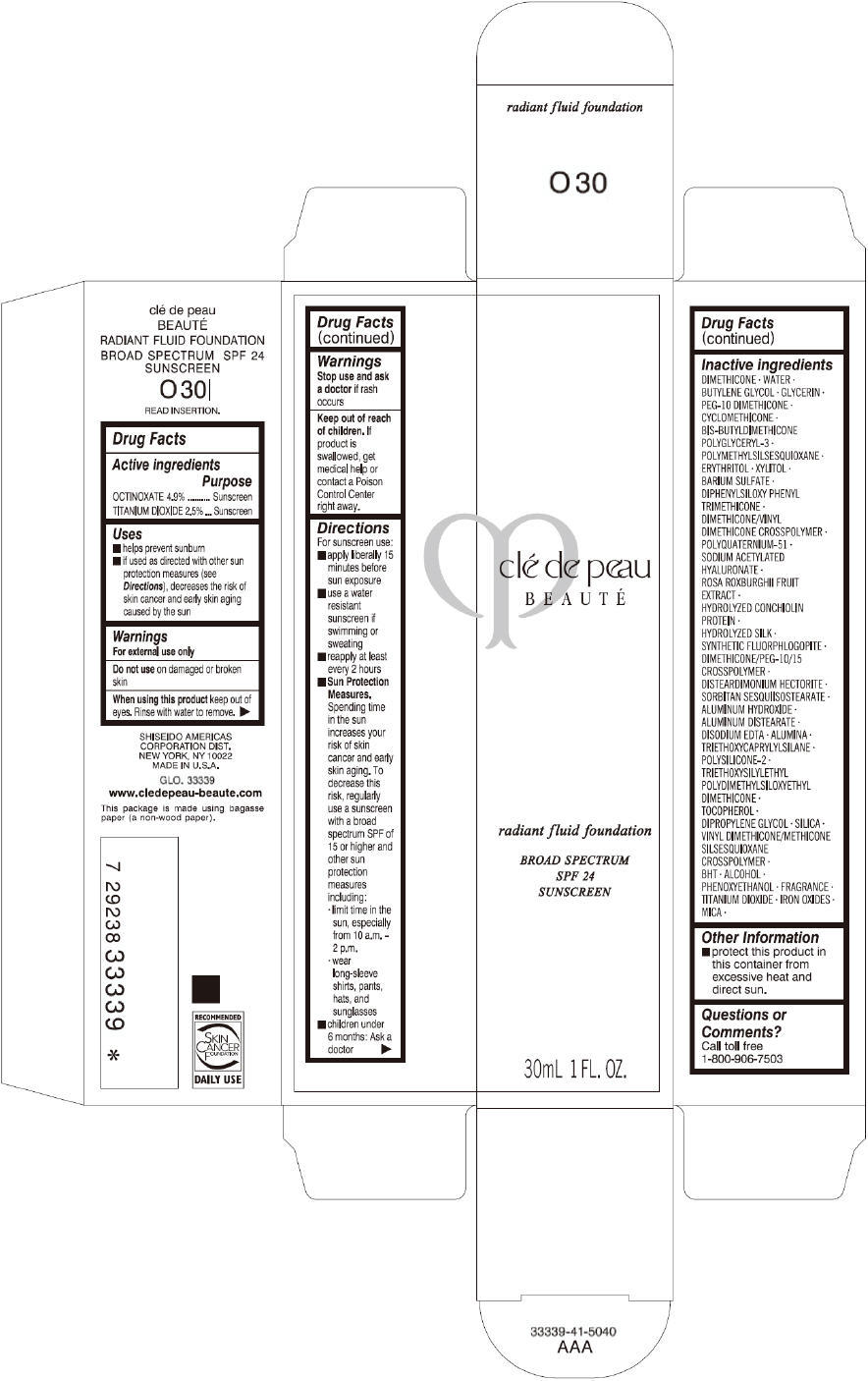
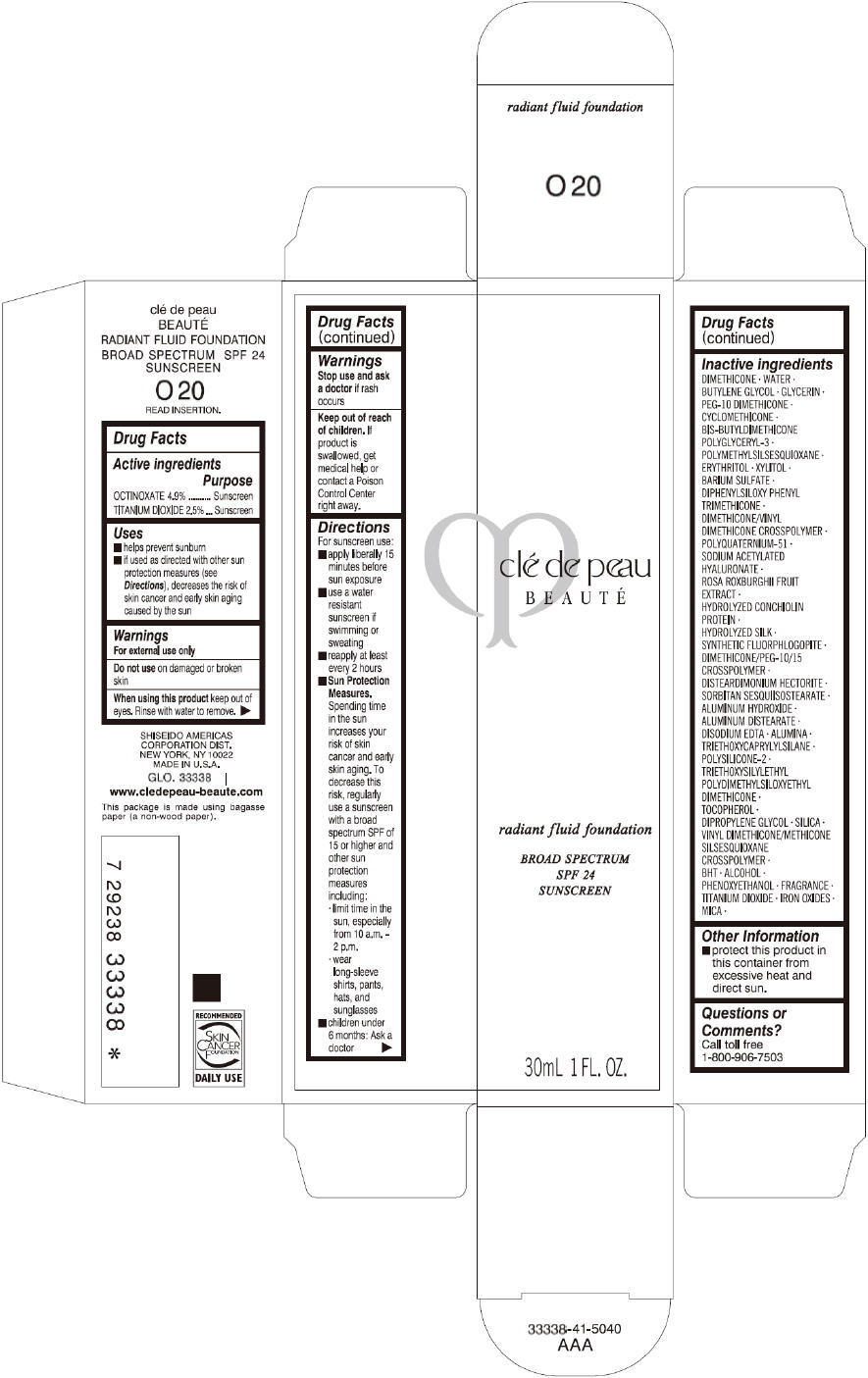
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
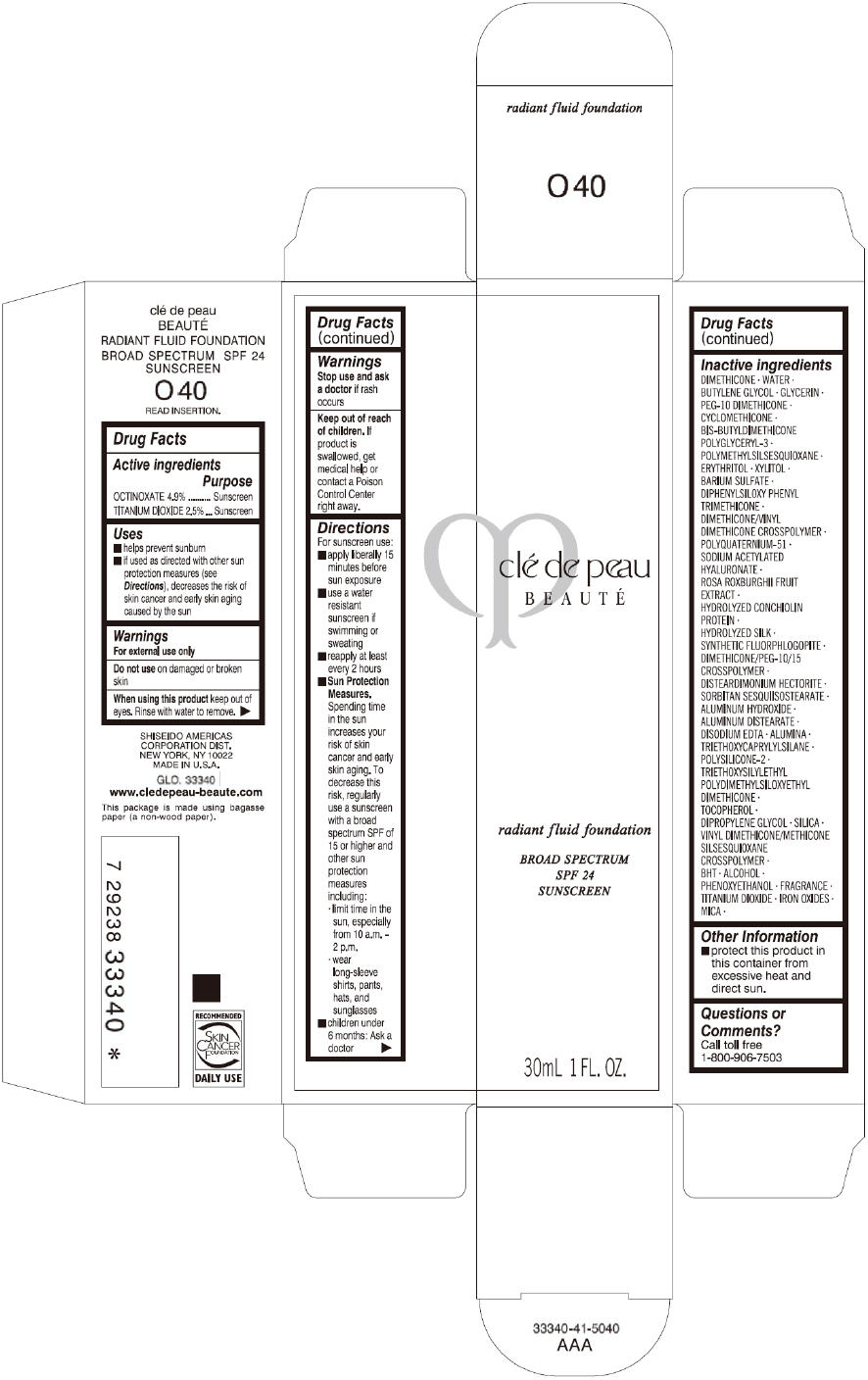
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
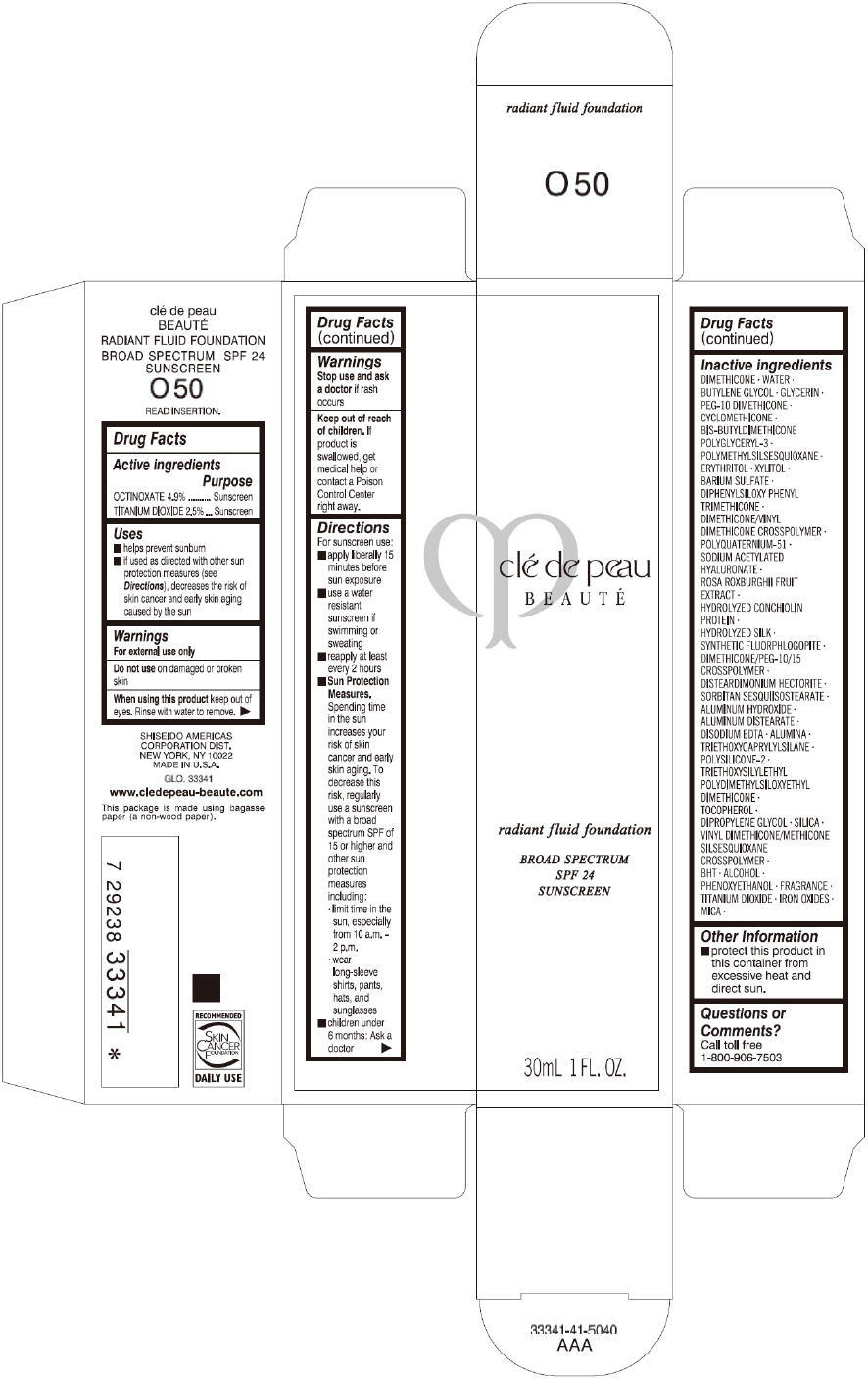
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
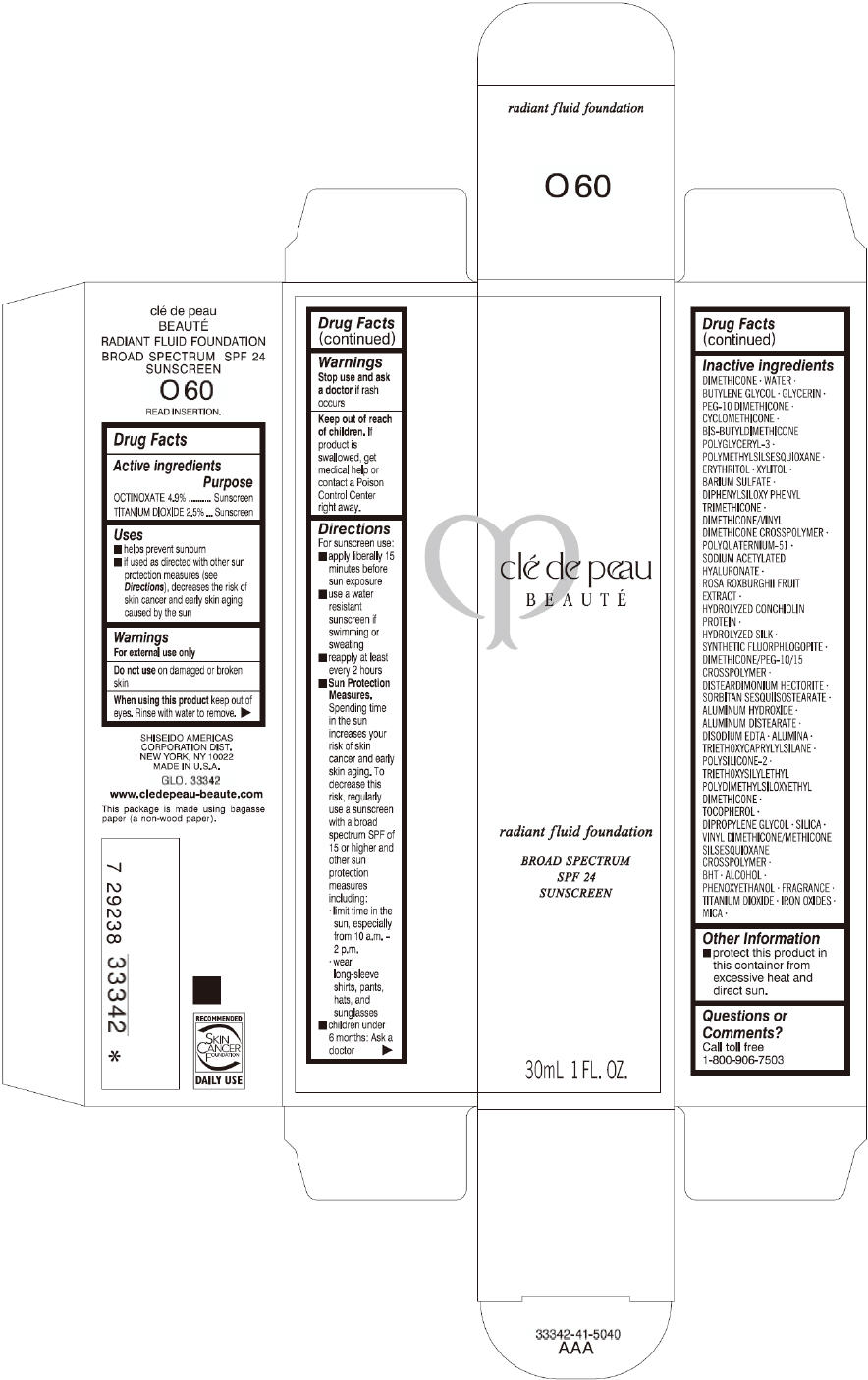
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
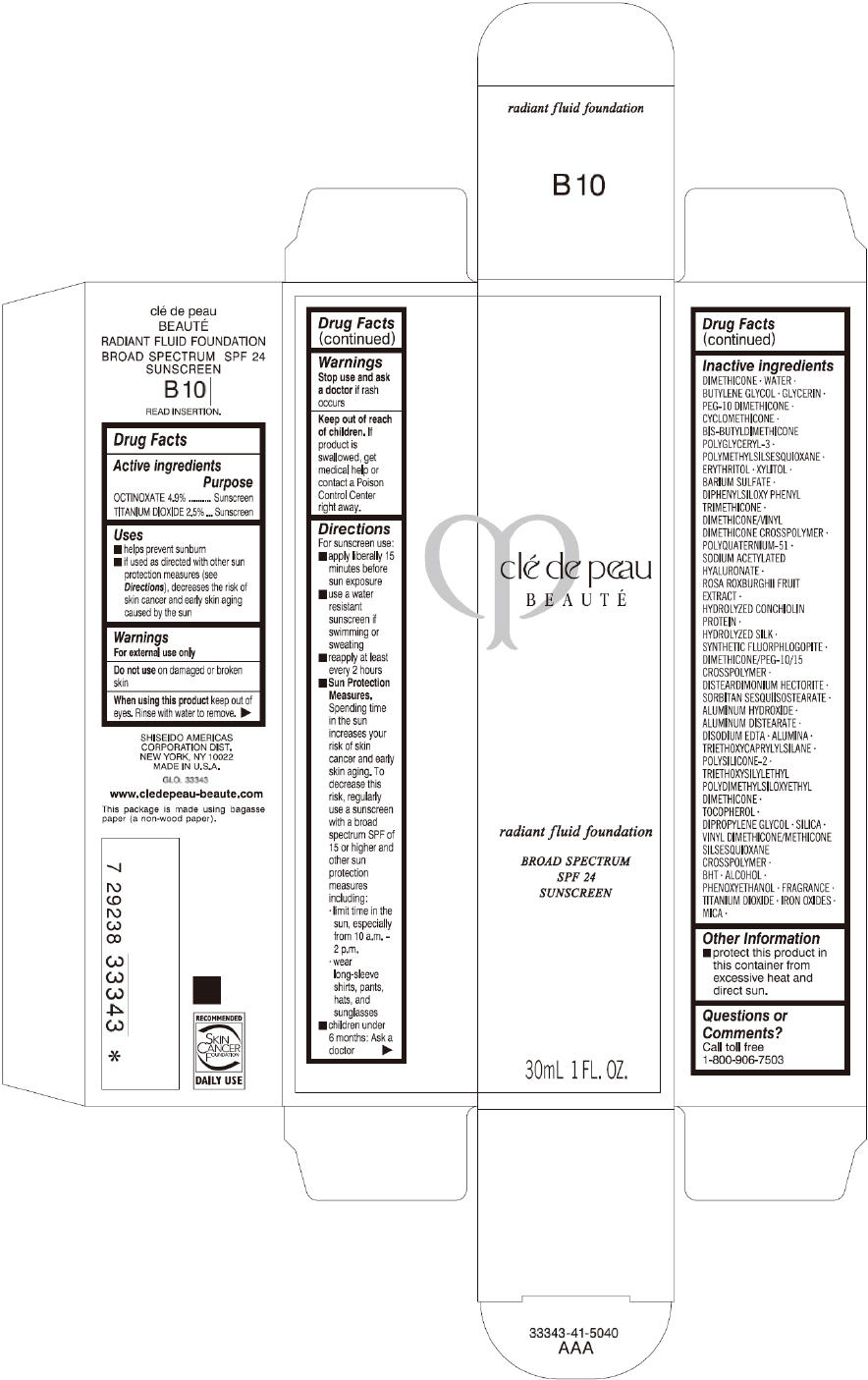
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
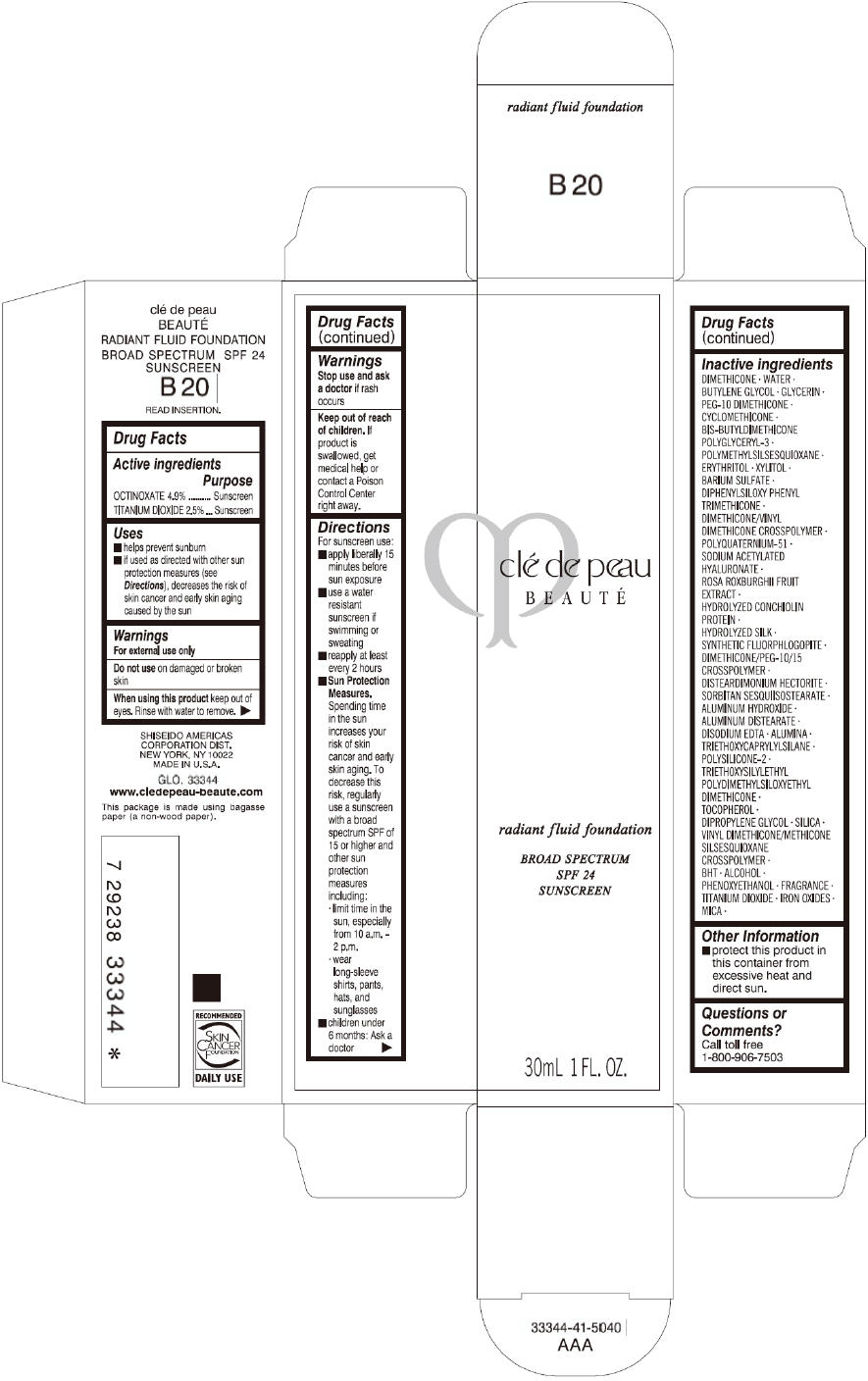
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
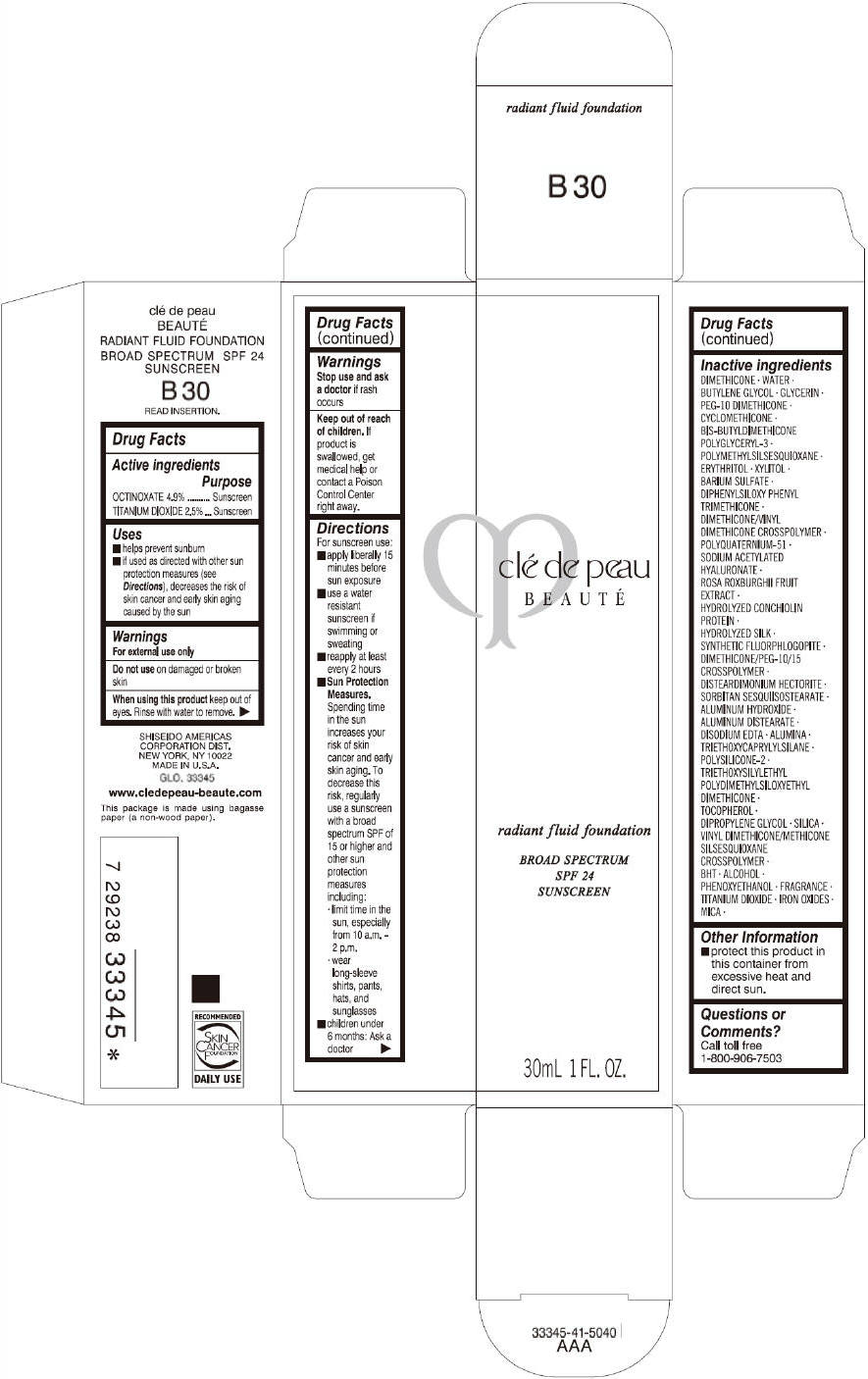
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.
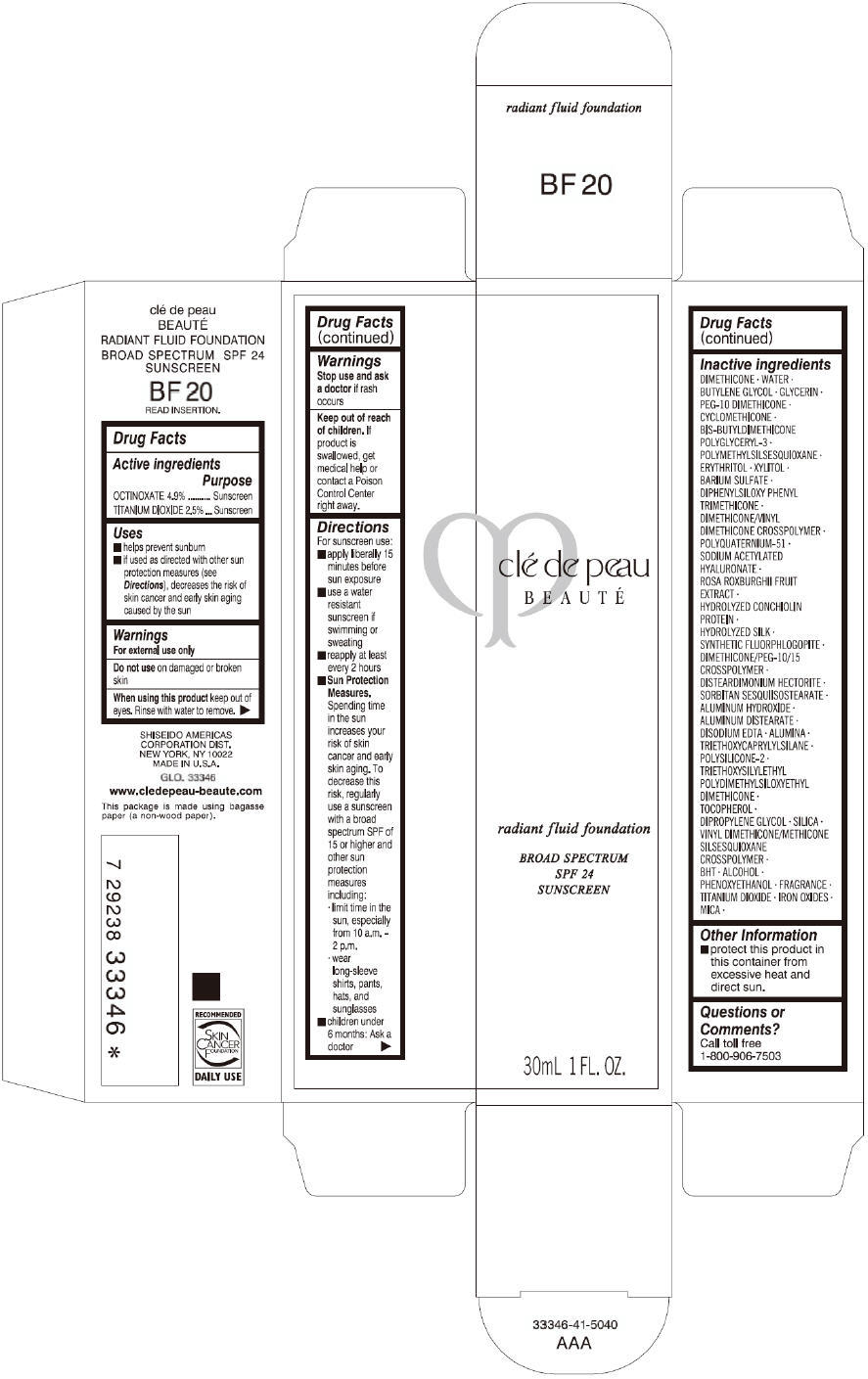
PRINCIPAL DISPLAY PANEL
clé de peau
BEAUTÉ
radiant fluid foundation
BROAD SPECTRUM
SPF 24
SUNSCREEN
30mL 1 FL. OZ.